
by Andrew Pasquale Tuesday, February 16, 2016
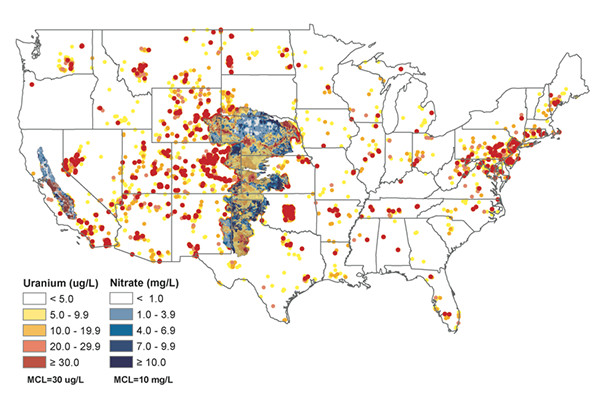
Uranium (red) and nitrate (blue) levels in groundwater are indicated at sites across the U.S. in this map, including in the Central Valley (California) and High Plains (central U.S.) aquifers. Credit: University of Nebraska--Lincoln
Nitrogen is an essential nutrient for plant life, but plants can only take up so much so fast. When excess nitrogen enters the environment by way of fertilizer and manure runoff, as well as in automobile and industrial emissions, it becomes a pollutant that can leach into waterways, carrying with it unintended — and often undesirable — consequences. In a new study, researchers have found evidence of one such consequence: elevated uranium levels in two major U.S. aquifers.
Together, the High Plains aquifer — which underlies portions of Colorado, Kansas, Nebraska, New Mexico, Oklahoma, South Dakota, Texas and Wyoming — and California’s Central Valley aquifer provide about 6 million people with drinking water and irrigate 56,700 square kilometers of cropland. Meanwhile, more than one million tons of nitrogen-based fertilizers are used annually on farms in these regions.
The study, published in the journal Environmental Science and Technology Letters, links nitrate — a form of oxygenated nitrogen commonly found in or formed from fertilizers — to uranium mobilization, “suggesting that nitrate groundwater contamination could also lead to uranium mobilization in groundwaters,” says Karri Weber, a biologist at the University of Nebraska at Lincoln and a co-author of the new study. Contamination of aquifers with uranium, which is known to impair kidney function and damage bones, could pose a public health threat, Weber and University of Nebraska graduate student Jason Nolan noted in the study.
Most work on uranium contamination considers anthropogenic sources from things like uranium mining and milling, nuclear weapons tests and disposal of spent nuclear fuel, Weber says. But natural uranium is present at low concentrations in soils derived from weathered igneous parent minerals such as uraninite and coffinite.
Weber and Nolan examined uranium and nitrate concentration data for 276,511 samples of groundwater and drinking water collected by federal, state and local agencies from nearly 62,000 sites above the High Plains and Central Valley aquifers. They then compared the levels to the Environmental Protection Agency’s Maximum Contaminant Limits (MCL), which are the legal limits for substances allowed in public drinking water. They found that uranium, which has an MCL of 30 micrograms per liter, exceeded this level in 10 percent of tested wells across a combined area of 22,375 square kilometers. Nitrate, meanwhile, exceeded its MCL of 10 milligrams per liter in 21 percent of tested wells over a combined area of 99,162 square kilometers. That means that approximately 1.9 million people could be impacted by uranium groundwater contamination in these two aquifers alone.
The researchers noted that in approximately 78 percent of sites where groundwater uranium concentrations exceeded the MCL, the contamination was moderately to strongly correlated with high nitrate levels, suggesting a link between nitrate and uranium contamination. After oxidation by nitrate, uranium can form chemical complexes with carbonate and calcium ions in groundwater, which allows it to move through the soil column and into the groundwater. The precise concentration of nitrate required to mobilize uranium is not known, Weber says, but a statistical analysis in this study suggests that “nitrate concentrations at or near the MCL … may be that point at which uranium will get mobilized.”
The relation between high nitrate and uranium levels is likely due to a process called oxidative dissolution. When it comes to aquifer waters, uranium comes in two forms — reduced and oxidized. “When uranium is present in rock, it is present in the reduced form, and if it’s oxidized it will be dissolved … out of the rock,” explains Lee Krumholz, a geomicrobiologist at the University of Oklahoma who was not involved in the study. “Once microorganisms begin to use the nitrate, then the nitrate can oxidize the uranium and the uranium will be dissolved,” Krumholz says.
Another finding from the study was that shallow wells sourcing water from less than 30.5 meters deep were eight times more likely to be contaminated than deeper wells. Weber says that this may be explained by how far nitrate must move through the soil column to reach groundwater. “The deeper [nitrate] has to go, the more opportunity that it could be scavenged either by an abiotic or biotic reaction. So it may be reduced before it gets to deeper portions of the aquifer,” she says.
The study raises “a really important problem,” Krumholz says, “in that it suggests that nitrate from agricultural runoff … could be releasing uranium into the groundwater.” He says there are plenty of additional locations for future research into this process, noting that there are many areas where nitrate might find its way into groundwater, “not just from agricultural runoff, but from municipal runoff as well.”
Weber says she plans to explore “both abiotic and biotic processes that are leading to uranium mobilization” underground to get a better understanding of the driving factors and the rates at which the mobilization occurs. This information could “then be utilized in predictive models to begin to estimate when or if it would become a problem,” she says, as well as to help “develop management strategies that might control or prevent uranium mobilization if an area is potentially at risk.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.