
by Nick Parkins Thursday, October 12, 2017

Naturally occurring corrosive water is not necessarily dangerous to drink, but it can become dangerous when it encounters lead or other toxic metals in pipes, solders and fixtures. Credit: ©iStockphoto.com/eldadcarin.
Lake Huron, situated between Michigan and Ontario, Canada, is the third-largest freshwater lake on Earth by area and one of the five Great Lakes that together hold some 23,000 cubic kilometers of freshwater. Most of the water in the Great Lakes originated as glacial meltwater, and the lakes are underlain by huge subterranean reservoirs in glacial deposits and underlying bedrock. These groundwater aquifers hold about 4,200 cubic kilometers of freshwater — approximately equivalent to Lake Michigan — and, along with the Great Lakes, help meet the water needs of millions of people. Until recently, Lake Huron supplied water to the city of Flint, Mich. However, in 2011, Flint city officials decided to switch the source of the city’s municipal water supply from treated water taken from Lake Huron to water from the Flint River — a decision that would have grave consequences for public health.
The Flint River has naturally high concentrations of chloride salts, which makes the water corrosive. Such water is fine to consume if treated properly. Federal law requires that municipalities treat corrosive source water with anti-corrosion agents before it is distributed through supply lines — which officials in Flint failed to do. In 2014, after the city began channeling its river water through aging lead service pipelines and lead-tainted pipes, joints and fixtures in people’s homes, complaints from residents about unusual odors, tastes and health issues — seemingly stemming from the tap water — multiplied. An outbreak of Legionnaires’ disease during this time caused at least 12 deaths. Subsequent tests run by scientists at Virginia Tech showed Flint’s water supply was 19 times more corrosive than water from Lake Huron, suggesting the city’s water could be leaching lead out of the pipes. And indeed, that’s exactly what scientists found; lead levels in one home reached 13,200 parts per billion (ppb), more than 2.5 times the level that qualified the water as hazardous waste, according to Environmental Protection Agency (EPA) standards. An investigation later showed that officials had, despite evidence that the water was contaminated, continued to allow residents to consume it. In June 2017, five local and state officials involved in the water crisis and its cover-up were criminally charged with involuntary manslaughter.
Flint residents are still experiencing the ill effects of exposure to lead, concentrations of which in the city water peaked at nearly 900 times the EPA action limit of 15 ppb — the level at which water managers are required to initiate additional corrosion-prevention measures, and to inform the public “about steps they should take to protect their health.”
The crisis in Flint raised questions across the country, especially: Could this happen here? Multiple factors contribute to whether water will become corrosive, but in many places, the answer is a qualified “yes.”
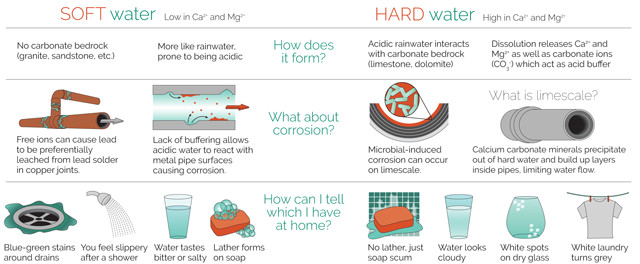
Rainwater is naturally acidic because it absorbs atmospheric components like carbon dioxide, which dissolves in water to form mild carbonic acid. Dissolved oxygen is also a corrosive agent in water, as are dissolved solids like salts and sulfates. Another factor that can increase corrosivity is water softness — a lack of dissolved water-hardening calcium and magnesium ions. In fact, the most common cause of corrosivity in groundwater, according to Ryan Gordon, a hydrogeologist at the Maine Geological Survey, is softness and low pH, which often go together. “Water that is low in calcium and magnesium lacks buffering against acid, so it is often more acidic,” Gordon says.
Understanding corrosivity is challenging. Often, physical factors, such as the rate of water flow and water temperature, complement and expedite chemical corrosion. And simply raising the pH doesn’t necessarily help; in some cases that can encourage corrosion. For example, mineral coatings, or scales, that form in pipes in response to alkalinity — which might otherwise help prevent corrosion — may help sulfate-reducing bacteria thrive, resulting in microbially induced corrosion.

Sampling a well in Shelby County, Tenn. Credit: Alan Cressler, USGS.
Surface waters are typically more corrosive than groundwater. But surface waters are typically treated for corrosivity by municipalities. So health issues associated with water corrosivity arise predominantly in areas where the water supply is sourced from groundwater. In the United States, groundwater accounts for 45 percent of the potable water supply overall, but aquifers supply 99 percent of the country’s rural communities, many of which rely on private wells that are unregulated and where monitoring is left up to private owners. That said, corrosive water and associated problems can be found almost anywhere.
In the wake of the Flint crisis, a study released in 2016 by the U.S. Geological Survey (USGS) National Water-Quality Assessment Project (NAWQA) revealed the potential corrosivity of groundwater nationwide. The report suggests that water supplies for tens of millions of people may be at risk of lead contamination from pipe corrosion.
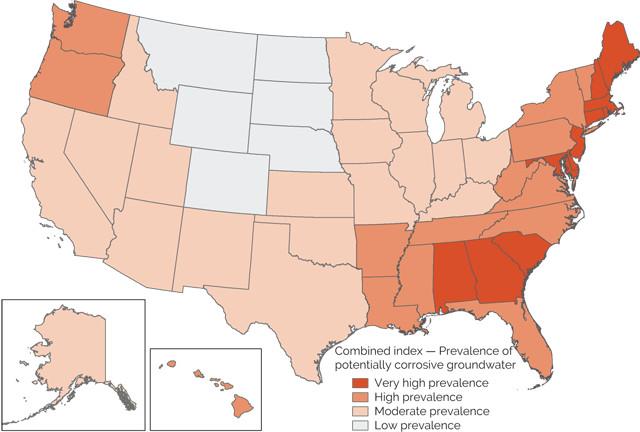
The 2016 report by the U.S. Geological Survey (USGS) National Water-Quality Assessment Project (NAWQA) revealed that the water supplies for tens of millions of people across 25 states may be at risk of lead contamination from pipe corrosion. Credit: USGS.
Approximately 44 million people in the U.S. use water from private wells and springs. “For the most part, the only testing done on this water is carried out by the homeowner, and this isn’t always regular or commonplace,” Gordon says. To understand corrosivity in untreated groundwater, USGS mapped well source water from more than 27,000 sites nationwide, including private and public wells, as well as springs. The data for the study spanned 24 years, and the study is the first of its kind, says Kenneth Belitz, a research hydrologist at USGS in Northborough, Mass. “There has been no national map prior to this work,” he says.
The researchers relied on two primary assessments of groundwater corrosivity: the Langelier Saturation Index (LSI), which assesses the potential for mineral deposition, and the Potential to Promote Galvanic Corrosion (PPGC), which relates potential corrosivity to an electrochemical process.
When calcium or magnesium carbonates are present, they can be deposited on the insides of pipes, forming protective scaling that inhibits corrosion of the pipe itself. The LSI is a measure of calcium carbonate saturation, which indicates the potential for scaling to occur. Calcium carbonate saturation depends on groundwater composition, including pH" alkalinity, temperature, and the concentrations of calcium and dissolved solids. Low LSI values mean water is sof", containing little calcium or magnesium. If no scaling occurs, water can be considered corrosive.
Galvanic corrosion of lead is an electrochemical process. It often occurs when lead plumbing components such as pipes or solder come into electrical contact with other metals, such as copper, in the presence of an electrolyte, or salt solution. The PPGC measures well water conductivity and signifies the corrosive potential of untreated groundwater. “If the water contains a lot of dissolved salts, or ions … it is more likely to conduct electricity,” Gordon says. “This factor is known to increase the corrosion of lead and copper in plumbing systems.”
Combining the LSI and PPGC indices, Belitz and his team mapped the potential for groundwater corrosivity across the country. The highest levels were found across 25 states in the Southeast, Northeast and Northwest, which, combined, are home to 24 million people who rely on water from private wells.
A primary reason some areas have more corrosive groundwater than others has to do with the varying geology of different aquifers. If aquifers “lack neutralizing capacity, the resulting groundwater will retain its acidity,” says Gary Rowe, program coordinator of the USGS National Water Quality Program, based in Denver, Colo.
Credit: K. Cantner, AGI.

Millions of people rely on water from private wells like this one. Credit: Callan Bentley.

Brian Selck, a USGS National Association of Geoscience Teachers intern, measures the depth to the groundwater in a domestic well in Pennsylvania that is being tested for corrosive water. Credit: Eliza Gross, USGS.

The Virginia Cooperative Extension Household Water Quality Program tests for hardness, lead and pH, along with a number of other constituents, including arsenic, copper, fluoride, iron, manganese, nitrate, sodium, sulfate, total dissolved solids and bacteria. Credit: all: Jim Stroup, Virginia Cooperative Extension.
Carbonate rocks, such as limestone or dolomite, neutralize acidity “and reduce the potential corrosivity of groundwater pumped from the aquifer,” Rowe says.
“In parts of Connecticut, Delaware, Maine, Maryland, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania and Vermont, the high groundwater corrosivity mapped by USGS is frequently explained by the relatively unreactive sandstone or granite bedrock aquifers that underlie these areas, Rowe says. “In such circumstances, the groundwater will be potentially corrosive if it is not neutralized prior to public or domestic use,” he says.
Pennsylvania is particularly prone to corrosivity issues owing to its aquifer geology and its high reliance on groundwater. The state has 30 times more freshwater stored underground than at the surface, ranking behind only Michigan in terms of the total number of wells and the number of household wells. In Pennsylvania, 1 million households receive drinking water from wells and springs tapped by public water companies, and an additional 1 million households (approximately 3 million people) rely on private wells and springs.
The U.S. Safe Drinking Water Act gives the EPA authority to set national drinking water standards. However, it only applies to water systems and wells that serve more than 25 people. Thus, maintenance of private wells — including testing and treatment of well water — is not regulated by states and is considered the responsibility of the homeowner. In Massachusetts, for example, more than 500,000 people rely on private wells; in Minnesota, that number surpasses 1 million. Wisconsin has at least 800,000 private wells, servicing 1.4 million people. In Virginia, where 1.7 million people rely on wells or springs, scientists at Virginia Tech tested a sample of these water systems. In a 2015 study, published in the Journal of Water and Health, researchers collected and tested more than 2,000 samples of “first draw” water — water that had remained in plumbing systems overnight — from private wells. The researchers found that in almost 20 percent of wells, lead concentrations exceeded the EPA action level.
Together, state-scale maps offer a regional picture of hazards from groundwater corrosivity. However, Rowe says, because aquifer rock type and soil composition vary widely within states, variations in corrosivity, and the resulting risk, occur on local scales.
Private well owners in susceptible regions are often advised to switch to public supplies — if available — on the presumption that treated water is better. In general, Gordon says, “public water systems monitor their water for corrosivity and other quality concerns, and correct them with treatment before sending water through the distribution system … although lead contamination does still occur.” If public water supplies aren’t available, then it is suggested that homeowners test their water every three years, he says (see sidebar).

Philadelphia uses water from the Delaware and Schuylkill rivers (the latter shown here). Waters in both are corrosive. Credit: Montgomery County Planning Commission, CC BY-SA 2.0.
“Although corrosivity tends to be more hazardous in private wells — where the water often goes untested and untreated — cities face their own challenges.
Philadelphia, for example, sits atop unconsolidated sand and gravel aquifers that lack soluble minerals that might otherwise buffer the natural acidity of precipitation. The city, which takes its water from the Delaware and Schuylkill rivers, has experienced corrosive contamination that has affected the city’s schools, with lead levels as high as 300 ppb measured at school drinking fountains, despite the city implementing corrosion-control measures at its water treatment facilities. In fact, tests conducted on school district water, around the year 2000, returned a lead spike of 16,000 ppb from one school bathroom faucet.

The water in Portland, Ore., is corrosive, which can cause problems with lead pipes and fixtures. Credit: Eric Prado, CC BY-NC 2.0.
On the other side of the state, Pittsburgh saw average lead levels in drinking water rise from 6 ppb in 2001 to 14.8 ppb in 2013. The rise preceded the decision in 2014 by the Pittsburgh Water and Sewer Authority’s former management firm, Veolia, to switch from using soda ash (a chemical similar to baking soda) to treat water corrosivity to a cheaper and less-effective alternative, caustic soda — a switch that was not disclosed to the Department of Environmental Protection. Soda ash is known to line pipes with a carbonate scale, which caustic soda does not do. Some reports suggest caustic soda may also cause any carbonate-scale lining that has built up to deteriorate, or cause corrosion of exposed metal pipes.By 2016, lead levels in 17 percent of homes were higher than the EPA’s action level. Pittsburgh is the second-largest municipal water system in the U.S. with lead levels that exceed the EPA’s action level.

The city of Portland gets its water from the Bull Run Reservoir. Credit: Eric Prado, CC BY-NC 2.0.
Portland, Ore., is the largest municipal water system with too-high lead levels. In Portland, lead levels in older homes (considered to be at high risk for lead) accessing the municipal water supply are the worst among the country’s 75 largest cities. Portland takes its water from the Bull Run Reservoir, which is nestled among the well-drained, loamy soils of the Bull Run watershed. The pH of loam is low, resulting in naturally corrosive surface and groundwater. In 1992, lead was found in Portland’s water at three times the EPA action limit. Five years later, the Portland Water Bureau started using caustic soda to treat its water. However, after high lead levels were found in 2013 and again in 2016, the EPA ordered the city to review its lead safety protocols and state regulators ordered the city to reduce lead levels. Portland Public Schools is also working with the city after tests revealed high lead levels in all but one of the 97 schools tested.

Lead levels were found to be too high in all but one of 97 schools tested in Portland in 2016. Credit: Sarah Mirk, CC BY-SA 4.0.
Following advice from the EPA, the Portland Water Bureau is considering adding soda ash and carbon dioxide to the Bull Run water to adjust the pH and water softness and reduce the corrosivity. In the meantime, other longer-term solutions are in the works. State regulators say this should include a new water treatment plant by 2022 that would permanently reduce lead levels.
The bedrock and soil beneath Portland may be the root of the city’s problem, but much of the city’s private infrastructure contributes, too. Although the city of Portland’s public pipelines do not include lead, many buildings have lead pipes, or have lead solders or fixtures. Aging lead pipes and solders are common in many cities across the U.S., especially in the Midwest and Northeast. In Chicago, for example, EPA tests revealed that roughly 80 percent of properties are serviced by lead pipes. In Milwaukee, some 70,000 lead service pipelines provide drinking water to residents; statewide, in Wisconsin, about 176,000 such pipelines exist.
Even in the wake of the Flint crisis, there’s no plan to replace most of the country’s lead service lines, mainly because it’s too costly: Digging up lead pipes in Milwaukee alone has been estimated to cost between $511 million and $756 million, according to Milwaukee Water Works. Nonetheless, a few cities, like Pittsburgh, have been ordered by state regulators to replace lines; Pittsburgh has to replace 7 percent of its existing public lead service lines each year until all lead service lines have been replaced, despite the cost of at least $411 million, according to the mayor’s office. Pittsburgh is also considering offering incentives to homeowners to replace their residential lead pipes.

USGS scientists sample water in a public supply well in Texas, overlying the Eagle Ford shale gas production area. Credit: Patty Ging, USGS.
There’s also the issue that there are few actual inventories of lead pipes in cities, and none across rural areas, which makes mapping and locating lead pipes a tough task. Currently, the EPA estimates at least 10 million homes and buildings in the U.S. are serviced, at least in part, by lead supply lines.

Acid mine drainage, such as in this tributary of Dutchman Run in Lycoming County, is a significant problem in parts of Pennsylvania. Residents with private wells near former mines are most susceptible to water problems associated with acid mine drainage. Credit: Nicolas A. Tonelli, CC BY 2.0.
Land-management practices can influence groundwater corrosivity. Permeable, unsaturated soils and rocks that lie above the water table can buffer groundwater from acidity induced by rainwater and pollutants as it filters through to the aquifer. However, when this filter is absent or inadequate, Rowe says, as in the poorly buffered shallow quartz sand aquifers common to the coastal plains of New Jersey, the use of agrochemicals, such as ammonia-based fertilizers, can result in acidification of shallow groundwater.
Human activities may also bypass the buffering capacity of soil. Belitz and his team have been assessing effects from hydraulic fracturing operations on groundwater quality amid unconventional oil and gas plays across the U.S. Faulty well casings and improper wastewater disposal at the surface offer potential routes whereby shallow groundwater can be contaminated, according to a study by Zacariah Hildenbrand of the Collaborative Laboratories for Environmental Analysis and Remediation at the University of Texas at Arlington and colleagues. However, contamination from deep oil and gas reservoirs into groundwater supplies — along fractures or faults, for instance — does not appear to have been a problem from hydraulic fracturing operations so far, Rowe notes.
“Another human-related influence on water corrosivity is acid mine drainage (AMD), produced as a byproduct of mining coal, gold, silver, copper, nickel, zinc, cadmium, iron and lead. One-third of all water affected by AMD nationwide is in Pennsylvania. Coal and metal ores often contain sulfides that oxidize when exposed to air. Water that filters through oxidized minerals forms acidic, sulfate-rich drainage that can pollute surface and groundwater. Residents with private wells who live near abandoned surface coal mines are most susceptible.
The use of road salt, predominantly sodium chloride, in the U.S. is also an issue because it is largely unregulated, which can result in sizeable amounts of chloride-rich runoff entering surface waters. Among the many environmental impacts of road salt, runoff can raise chloride-to-sulfate ratios in water supplies — similar to what happened in Flint. “This increases the potential for galvanic corrosion in systems that have metal pipes that are exposed to increasingly saline groundwater,” Rowe says.
As the corrosivity of our water supplies continues to cause problems, the demands we’re placing on these supplies continue to grow. USGS and other agency data can be used to make decisions about how best to protect and manage groundwater to meet current and future needs, Rowe says. Local, state and federal authorities can support this process and enforce transparent monitoring procedures to quantitatively assess what is an ongoing issue. And this is where things can get murky.
Lead testing methods are inconsistent, and results can be distorted. For example, running a cold tap for a couple of minutes prior to testing can flush out high concentrations of lead that might have dissolved and built up in water lines not used for some time. When employed as part of a daily routine, this method can help reduce a consumer’s exposure to lead. However, authorities in some cities have at times promoted this method to residents tasked with collecting samples to be tested for lead. The practice, known as pre-flushing, can obscure hazardous lead spikes. Use of pre-flushing prior to samples being taken has been recorded in major cities throughout the eastern U.S., according to a Guardian investigation.

The only way to know if there's lead in drinking water is to test at the tap: Virginia Tech's Erin Ling collects water for testing at a home with a private well in McCoy, Va. Credit: Logan Wallace, Virginia Cooperative Extension.
What constitutes a safe lead concentration in water is also unclear. The Centers for Disease Control and Prevention recently changed its standard to indicate there is no safe exposure limit to lead, advice that runs somewhat contrary to the EPA’s 15-ppb action limit and the 10-ppb standard set by the World Health Organization. The EPA limit, furthermore, is three times what the FDA applies to bottled water.
So, should the focus be on water supplies, pipes or both? “Certainly infrastructure with lead in the plumbing is concerning, even if the water is not unusually corrosive,” Gordon says. “In an ideal world, all lead plumbing would be removed and replaced carefully and responsibly, with the most sensitive populations — schools, homes with children — targeted first.”
In the meantime, regular testing of private well water is imperative in preventing exposure, Gordon says, even in cases where lead contamination isn’t expected. “Testing of private well water is so important because none of the potential known health risks, such as corrosivity, can be completely predicted or prevented,” he says. This also includes potential contamination by bacteria, arsenic, radon, uranium, lead and nitrates. “Most groundwater in areas that the USGS study highlights probably won’t require treatment, but the only way to know for sure is to test the water. Then the decision about whether to treat or replace plumbing can be made on a case-by-case basis,” he says. For now, he says, “the best thing to do is test, test, test.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.