
by Timothy Oleson Monday, February 2, 2015
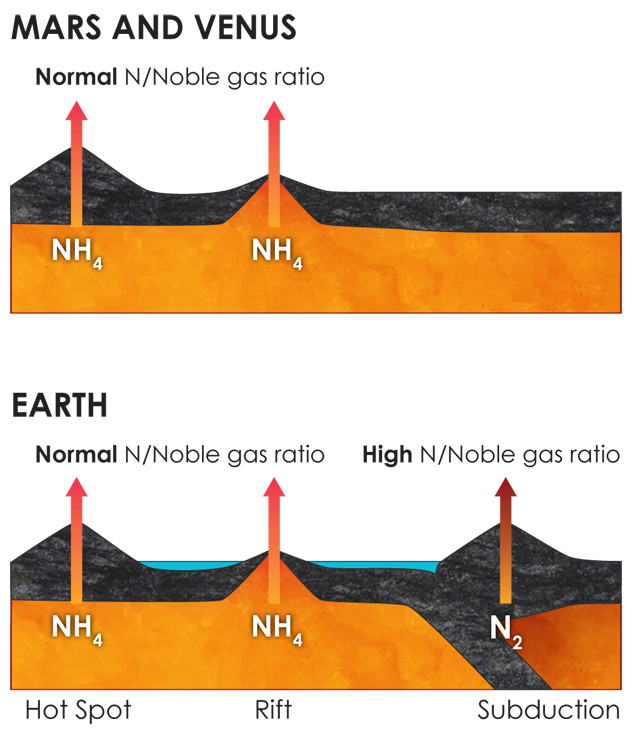
The chemically oxidizing conditions in subduction zones might explain the enrichment of nitrogen (and high ratio of nitrogen to noble gases) in Earth's atmosphere compared to those on Venus and Mars, neither of which has active plate tectonics. Credit: Kathleen Cantner, AGI, after Sami Mikhail.
Plate tectonics underlies many of Earth’s distinctive features, from its ever-shifting continents to its colliding mountain ranges and continuously forming crust at mid-ocean ridges. According to a new study, the process might also explain another of our planet’s peculiarities: its nitrogen-rich atmosphere.
Nitrogen, much of which has been belched into the skies from the planet’s interior by way of volcanoes and vents at the surface, accounts for about 78 percent of the planet’s atmosphere. This is a far higher share than in the atmospheres of Mars and Venus, both of which are dominated by carbon dioxide. (Venus’ atmosphere, because it is so much more dense than Earth’s — about 50 times more so at the surface — actually contains more nitrogen overall.) Compared to the atmospheres of our rocky neighbors, the ratios of nitrogen to noble gases like argon, neon, krypton and xenon are also higher on Earth. But considering that all three planets are thought to have mantles of similar composition, and that each planet shows evidence of volcanism, how the nitrogen disparity arose has remained unclear.
Addressing this question has been difficult because scientists have not had a firm grasp on what form, or species, nitrogen prefers to take when it is dissolved in fluids in the mantle at extreme pressures and temperatures — be it the molecular nitrogen that suffuses our air (N2), ammonia (NH3) gas or ammonium ions (NH4+). Calculations of such nitrogen speciation “have traditionally been limited to an upper pressure of 0.5 gigapascals,” well below mantle pressures, says Sami Mikhail, a geochemist at the Carnegie Institution of Washington and lead author of the new study in Nature Geoscience.
In the new study, however, he and co-author Dimitri Sverjensky of Johns Hopkins University took advantage of improvements in the understanding of water’s properties, on which nitrogen speciation depends, at pressures and temperatures up to 6 gigapascals and 1,200 degrees Celsius. These improvements, which came out of separate work led by Sverjensky, allowed them to model nitrogen’s behavior within the mantles of Earth, Mars and Venus.
They found that, under the chemically reducing conditions found in most of Earth’s mantle, as well as in Venus’ and Mars’ mantles, nitrogen should be present predominantly as ammonium, which can be absorbed by silicate minerals and does not tend to bubble out of water easily. But, they reported, within the portions of mantle above subducting tectonic plates, called wedges, where oxidizing conditions prevail thanks to the water pulled down with the plates into subduction zones, molecular nitrogen is the preferred form. Unlike ammonium, molecular nitrogen does not dissolve in rock, and when the pressure keeping it in solution is released, it readily bubbles out of water.
Mantle flow, the researchers wrote, would have “brought deep material containing ammonium in mafic minerals into the mantle wedge[s], where it can be efficiently degassed as nitrogen” through overlying volcanoes. Over geologic time, this would have amounted to quite a lot of the gas emitted into the atmosphere from subduction zones. In contrast, far less nitrogen is degassed through volcanoes and vents at Earth’s mid-ocean ridges or above mantle hot spots. Neither Mars nor Venus has active plate tectonics or subduction zones; relatively little nitrogen is pumped into their atmospheres through volcanism.
Other explanations have been offered for Earth’s nitrogen-rich atmosphere. If planetary size mattered, for example, Earth and Venus should have similar nitrogen enrichments, leaving diminutive Mars as the outlier. Or, if the giant impact thought to have formed Earth’s moon were to blame, then ratios among the noble gases — the ratio of argon to xenon, for example — should vary between Earth and its neighbors. And yet, as the researchers reported, these ratios are similar among all three planets. Mikhail and Sverjensky suggest that none of these alternate explanations fit the bill as well as subduction zone degassing.
Not only might the nitrogen puzzle be solved, but there are other implications too, Mikhail says. “Oxidizing ammonium produces nitrogen gas and water. If Earth’s nitrogen gas enrichment … is the product of subduction, then this also implies a proportion of the water on the surface is the result of subduction.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.