
by Jeffrey A. Karson and Robert J. Wysocki Friday, August 17, 2012
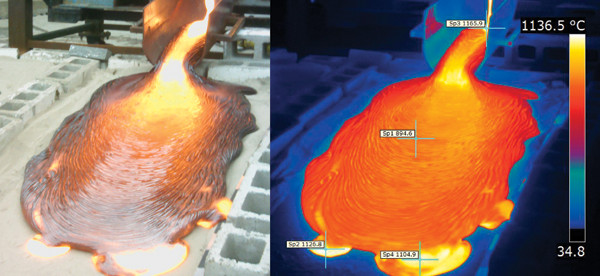
Pouring basaltic lava at Syracuse University's Comstock Art facility: Sheet-like, ropey pahoehoe flows over dry sand; infrared image of pahoehoe flow experiment with selected temperatures in degrees Celsius.
Melting a batch of the ancient basalt takes about four hours, but we hold the lava above its melting point for much longer to ensure that it is completely melted and to remove unwanted volatiles such as water. The lava is then poured at temperatures of 1,100 to 1,350 degrees Celsius, comparable to eruption temperatures of natural lava. We monitor it with a spot calorimeter and a Forward-Looking Infrared (FLIR) camera, the same instrument conventionally used at lava flows in the field.
We control the flow rate manually by carefully tilting the crucible and channeling the lava through a steel chute that’s about 20 centimeters in diameter. The movable chute permits multiple side-by-side lava flows to be poured from a single batch of lava.
All experiments are recorded on video — some of which are posted to the web (see http://lavaproject.syr.edu) — allowing for the calculation of flow rates and viscosities after the fact. Additionally, flow surface features often evolve in complex ways, making it difficult to determine how the flow developed from its final form alone.
After cooling (usually 12 to 24 hours), the flows are photographed, dissected and sampled. Crystallinity, vesicularity, and other textural features can be documented and correlated with flow morphologies.
At low flow rates, we have occasionally observed episodic inflation and downstream (or lateral) breakouts, which result in complex, overlapping lobes, pillows and secondary flows. Post-cooling dissection revealed discontinuous lava tubes with ceiling drips and sidewall drain-out features similar to those found in natural systems.
Most lava produced is glassy and black with varying densities of vesicles (trapped gas bubbles), a feature produced from moisture vaporized on the pouring surface. Samples of lava taken directly from the crucible do not have measurable amounts of dissolved gasses or bubbles. The bubble-lava interactions can help us better understand the role of vesicles in natural lava flows.
In many of the experiments, we saw tiny (1-millimeter) needle-like plagioclase feldspar crystals form in well-insulated pockets of relatively thick (about 10-centimeter), low-temperature (about 1,100 degrees Celsius) flows. It is not clear if the crystals formed during active flows or subsequent cooling. Experiments with tiny, 1-millimeter-diameter stainless steel beads, meant to simulate crystals, are planned to determine the influence of such particles on lava viscosity and flow morphology.
We produce different lava flow features by varying the pouring (effusion) rate and temperature. We also vary the features by changing the slope and texture of the substrate on which they are poured.
In one series of experiments, we poured the flows over dry sand surfaces inclined at 5 to 20 degrees. In these flows, the behavior and resulting morphologies are predictable and systematic, ranging from sheet-like pahoehoe to tube-fed and inflated forms. Large volume flows (400 to 500 pounds) poured at relatively low effusion rates over gentle slopes (5 to 10 degrees), for example, result in lobate to ropey flows with hummocky upper surfaces typical of pahoehoe flows. These flows developed through the growth of overlapping bulbous lobes at the distal end of the flow, similar to flow architecture at various scales in flood basalts.
At higher flow rates or steeper slopes, a channel forms due to the buildup of cool, viscous lava on the edges of the flow. The channel feeds an elongated, sheet-like pahoehoe flow. By holding the temperature constant and varying the slope (or vice versa), we hope to determine the transition points between these different morphologies.
Flows over moist or wet sand generate large vapor bubbles (limu) that are tens of centimeters in diameter in some cases. In one flow, a very large single, 30-centimeter-diameter bubble formed, expanded and ultimately cracked and collapsed upon cooling. The result was a circular hole in the base of the flow with a raised (1-centimeter) rim exposing the underlying sand surface. Bubble fragments littered the interior. Overall, this resembled a small “rootless cone” or “pseudocrater,” typical of where lava has flowed over wet soil or permafrost.
During some experiments, we have had to change pouring spouts between test runs. Moving these heavy steel channels with hot lava stuck to them has resulted in masses of thread-like Pele’s hair that form as the spout is pulled away from the crucible, stretching the lava into long strands. Individual strands as thin as human hair and up to several meters long are remarkably flexible and strong. In some cases, broad sheets of lava are also stretched like taffy to a brownish translucent film. As it cools, it transforms from a sticky sheet to a brittle pane of “dirty glass.”
Pouring lava down an inclined rock slab and into water in a large stock tank resulted in a number of surprises. Lava behaved as usual on the rock surface until it reached the water. Grapefruit-sized bubbles enclosed by highly fragmented lava (hyaloclastite) formed at the edge of the water and then floated out into the water like small rafts. As the bubbles expanded and burst, the rafts sank and piled up in clumps on the bottom of the water tank.
Lava poured into a J-shaped ceramic tube and extruded 30 centimeters underwater formed highly fragmented lava that rapidly welded into a fragile mass of glassy shards. These materials resemble much more altered hyaloclastites common on seamounts and in subglacial environments. The hyaloclastite formed quiescently, without any violent activity, contrary to expectations from natural examples.
Lava interacts with ice near the ice-capped summits of volcanoes in high-latitude settings such as Iceland. Lava has also probably interacted with ice — both water-ice and carbon dioxide-ice — on other planetary bodies in the past. Distinctive lava forms may provide evidence of lava-ice interactions from the distant past. We wanted to see what it would do in our “lab.”
In experiments with lava poured over ice surfaces, highly vesicular flows developed, exhibiting dynamic formation, growth and bursting of up to soccer ball-sized bubbles. The bubbles cooled rapidly, so they could be examined immediately. Students have been amazed to find that the bubble walls are often flexible and transparently thin, much like plastic food wrap. Seeing the progression of solid rock to molten lava to such a thin, flexible, exotic material is stunning.
A number of our experiments on ice sheets and ramps have shown fascinating lava-ice interactions. Lava poured at a high effusion rate down a 20-degree water-ice slope resulted in dramatic hydroplaning of the bubble-rich lava flow that slid rapidly off the ice, demonstrating a potential hazard: If someone were to encounter this in the wild, it would be harder to avoid than more slowly flowing lava. Pouring the lava onto a horizontal carbon dioxide-ice surface demonstrated a similar hydroplaning effect in which lava rapidly splashed off the surface. Our ice experiments have direct applications for understanding the behavior and hazards of lava flows either in contact with glaciers on Earth or on other planets.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.