
by Cortney Cameron Tuesday, September 29, 2015

The cloudy outer coating surrounding this gem-quality diamond core contains tiny inclusions encasing fluids trapped when the coating formed at a depth of about 200 kilometers. Credit: Anetta Banas
Subducting oceanic plates that dive hundreds of kilometers beneath Earth’s surface carry with them cargoes of sediment and seawater. As the plate heats up the deeper it sinks, this seawater not only initiates melting in the rock above it, but can also trigger diamond formation, suggest the authors of a new study in Nature.
Before climbing to Earth’s surface by way of explosive volcanic eruptions known as kimberlites, certain types of diamonds, called fibrous diamonds, precipitate out of dense, water-rich fluids at depths exceeding 200 kilometers in the thick, rocky lithosphere that underlies the oldest parts of continents. These fluids are commonly encased in tiny bubbles, or inclusions, in these fibrous diamonds, which are composed of individual linear crystals that grow together rapidly. On their trip to the surface, the sturdy diamonds resist alteration from extreme changes in pressure and temperature, and shield their inclusions.
Thus, the diamonds “provide pristine snapshots of the deep earth that we can’t get any other way,” says Graham Pearson, a geochemist at the University of Alberta and a co-author of the new study. Although scientists have learned about these fluids’ compositions by analyzing the inclusions, identifying where the fluids originate has been more difficult.
Fibrous diamond high-density fluids fall into four categories based on their chemical compositions: saline, silicic and two different carbonate-rich compositions (low versus high magnesium). “Over the past 20 years, a few models were developed [to describe] the relationships between the compositions,” says lead author Yaakov Weiss, a geochemist at Columbia University’s Lamont-Doherty Earth Observatory, “but none were very strong.”
To investigate the relationships and origins of the fluids, Weiss and his team performed detailed chemical analyses on fluids extracted from 11 fibrous diamonds mined from Canada’s Northwest Territories. In some of the fluids, they found the “chemical fingerprint of seawater interaction with the oceanic crust,” Weiss says: high chlorine levels, positive europium to strontium anomalies and specific strontium isotopic compositions. By comparing a variety of geochemical traits — including silica, magnesium and rare earth element contents — of the saline fluids to other fluids from the diamonds in this and prior studies, Weiss’ team traced how one composition evolved to another. “What we show now is that the saline composition is parental to the other compositions, and that seawater is parental to the saline fluids,” he says.
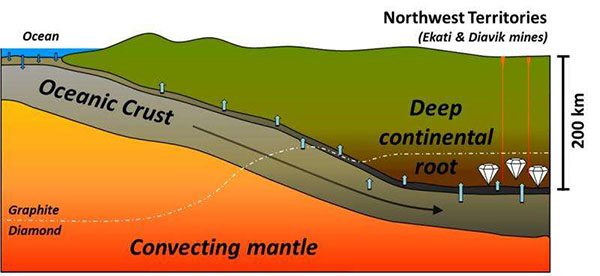
This simplified model shows how seawater can be carried in subduction zones to the base of continental cratons, where it can lead to the formation of diamonds. Credit: Y. Weiss et al. Nature, 2015.
In the researchers’ scheme, when these seawater-derived high-density saline fluids escape the subducting slab at depths exceeding 200 kilometers — where pressures become intense enough for diamond formation — they are squeezed and filtered into the continental lithosphere. “There, they interact with the rocks and initiate melting and fibrous diamond formation,” Weiss says.
Meanwhile, by comparing the strontium isotopic composition of the saline fluids extracted from the fibrous diamonds in the study to records of seawater strontium compositions, which have varied over geologic time, the researchers also found that the diamonds formed less than 200 million years ago in the Mesozoic Era. During the Mesozoic, several oceanic plates, including the Farallon Plate (survived today by the Juan de Fuca Plate off the northwestern U.S.), were subducting beneath western North America — where the study’s diamonds were found. The finding suggests that, unlike most of the Canadian gem-quality diamonds that are typically estimated to have formed billions of years ago, the fibrous diamonds formed much more recently.
Pinpointing seawater as the source of diamond-forming high-density fluids could help settle a long-running debate about the way that volatiles, particularly water and possibly carbon, are cycled through the deep earth and then back into the crust and mantle, Pearson says. “These diamond inclusions clearly show that water-rich fluids are directly cycled down to great depths, at least 200 kilometers, via subducted slabs — and some of it is streaming off into the root of the deep continent.”
Pearson, who led a 2014 study announcing that the mantle holds abundant water locked up in the common mantle mineral ringwoodite, adds that the current study provides “strong evidence” that the water in ringwoodite “is getting down to the [crust-mantle] transition zone by plate tectonic recycling.”
The study has additional implications for our understanding of mantle processes, particularly in that it shows the importance of saline fluids in the lithosphere, says Oded Navon, a geologist at the Hebrew University of Jerusalem who wasn’t involved in the new work. “Such fluids were previously ignored, but their presence may be important for the interpretation of mantle conductivity data,” Navon says. “The same is true for the interpretation of mantle trace element patterns, metasomatism [chemical alteration of rock by fluids] and much more. For example, what are the effects of saline fluids on the mantle’s mechanical strength?” he says. “Nobody knows.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.