
by Nicola Jones Monday, January 23, 2012
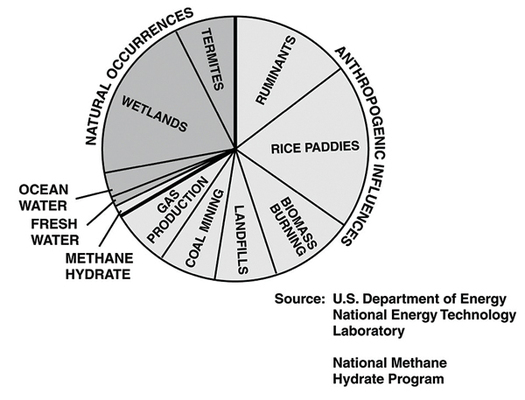
Estimated contributions of methane emissions in the U.S. Such emissions ratios are hard to tease out. NASA GISS

Doug Rumble of the Carnegie Institution of Washington stands behind a mass spectrometer. Doug Rumble
UCLA geochemist Ed Young in his lab, where he's working on sourcing methane using isotopologues. Ed Young
Ten years ago, John Eiler couldn’t convince anyone to build him his dream machine. He wanted a mass spectrometer that could measure the mass of common gases with extreme precision and sensitivity, but such a device would cost more than a million dollars and might not find a market: The companies that could make it didn’t think they would be able to sell more than just the one to Eiler, which didn’t make it worth their while. Even Eiler didn’t know exactly what problems he could solve with the device, though he had a hunch it would be useful. “At that time it felt so outlandish,” says Eiler, a geochemist at Caltech. “There were no examples of someone solving a scientific problem this way. So they basically all said ’no.'”
Science is lucky that Eiler didn’t give up. After finally convincing one company to modify a standard gas-source mass spectrometer — a cheaper option he hoped would prove his hunch — in 2003, he developed a technique that uses carbon dioxide as a kind of time-traveling thermometer to reveal temperatures from long ago. In the past few years, the technique has been used to take the body temperature of woolly mammoths and dinosaurs, and to pin down highly disputed temperatures of the ocean hundreds of millions of years ago. Now he has convinced a company to make something closer to his original dream machine, with which he hopes to tackle other molecules — including methane — and a whole new set of problems related to identifying the origins of those gases.
Methane has many different sources. The methane in natural gas reservoirs might be generated by the decomposition of organic matter buried deep underground long ago, by bacteria that munch on those organics, or by chemical reactions in rocks. The methane in our air is leaked from natural gas wells, spewed out from landfills, burped out by cows and burbled up from thawing permafrost in the Arctic. The methane on Mars, like that on Earth, might be geological or biological. Find a way to determine which methane is which, and you can answer questions about how fast permafrost is melting, how long natural gas deposits will last, and even whether there is life on other planets.
Eiler has some competition in his quest. While he tests his machine, another set of scientists has commissioned an even bigger and, they hope, better gas-source mass spectrometer with the same goal. Geologist Doug Rumble from the Carnegie Institution of Washington and geochemist Ed Young from UCLA have commissioned a brand-new $2 million machine as part of the Deep Carbon Observatory project — a mission, funded by the Sloan Foundation, to work out how much carbon is underground, how it got there, and where it’s going. Rumble and Young hope their machine, now in a friendly race against Eiler’s, will help to settle those same questions for methane.
For science, it won’t matter who gets there first. The benefit will be in the discovery.
The concept behind both machines is simple: They look at isotopologues. These are molecules that are chemically identical but contain different isotopes — atoms of the same element that have different weights. Most carbon, for example, has an atomic weight of 12, but a small percentage of carbon atoms have an extra neutron and weigh 13 atomic mass units. Molecules of carbon dioxide can contain normal carbon-12 or heavy carbon-13. These two varieties of carbon dioxide molecules are isotopologues.
Today, researchers interested in knowing the origins of methane first break the molecules apart, and run their constituent atoms through a mass spectrometer to determine the ratio of carbon-12 to carbon-13 or hydrogen to deuterium. Each of these values is a clue. Heavier isotopes tend to form more stable bonds, and this affects the kinetics of many reactions. Methane produced by microbes, for example, tends to contain very little carbon-13. This is because the bugs find it easier to metabolize weaker-bonded carbon-12 when they eat, and so they have less carbon-13 in their systems.
But there is only so much information you can glean from these numbers. The range of isotope values typical for one source of a gas tends to overlap with the range for another source, making different interpretations of the results possible. For example, Young says, “you can make methane abiologically in the lab that has the carbon-13 signal of biogenic methane.” This makes it impossible to definitively finger the sources of many molecules. “We’re not entirely in the dark. But we’ve realized we can’t do it with the tools we have,” says Barbara Sherwood Lollar, geochemist at the University of Toronto in Ontario, Canada, who is on the advisory council for Young and Rumble’s effort.
For decades, researchers have been using the isotope ratio of oxygen in carbonate seafloor rock, made of the remnants of shelled organisms, as a thermometer to read the temperature of ancient seas. The colder it was when these shells were deposited, the more oxygen-18 they tended to incorporate. But there’s a problem. The proportion of oxygen-18 in the rocks was also affected by the concentration of that isotope in the ocean, which in turn is affected by the amount of ice at the poles.
As water molecules journey from the equator toward the poles, the lighter isotopes (containing oxygen-16) preferentially evaporate from the sea and the heavier ones (containing oxygen-18) preferentially rain down; thus the water that eventually falls as snow is the most isotopically light on Earth’s surface. So the more ice there is at the poles tying up oxygen-16, the greater the quantity of oxygen-18 in the sea. Although researchers can get very detailed numbers about the oxygen-18 values of the rocks, it is uncertain exactly what those numbers mean: Was that part of the ocean cold, or were there large ice caps at the poles, or both? “It’s precise, but inaccurate,” Eiler says. “In the deep geological past, it gets sort of hopeless.”
Eiler says he knew that a better thermometer could theoretically be made by looking at molecules with more than one isotopic substitution, such as carbon dioxide made with both carbon-13 and oxygen-18, or methane with carbon-13 and deuterium. These “clumps” of heavy isotopes are very rare, but have very stable bonds. The proportion of clumped isotopologues in seafloor rocks depends not on how much of the stock material (like oxygen-18) is around, but almost entirely on the temperature at which they form: When the environment is cold, the ultra-stable bonds are favored. Look at the “clumped isotopologues” and you don’t have the same problem that you have with oxygen-18 alone: You can tell the temperature of the local waters, regardless of how much ice was around at the poles.
Measuring clumped isotopologues is tricky, however, because so many of them have such similar masses. Methane made of carbon-13 and one deuterium atom weighs about 18.041 atomic mass units, for example, whereas methane made of carbon-12 and two deuterium atoms weighs about 18.044. The best high-precision gas-source mass spectrometer in use today can resolve about 1/900 of the mass it’s looking at, so it can only tell 18.04 from 18.06 at best.
Back in 2003, Eiler convinced Thermo Fisher Scientific to make him a machine capable of distinguishing the one clumped isotopologue of carbon dioxide that’s relatively common in nature: carbon-13 with one oxygen-18 and one oxygen-16. “It was a teeny tweak to existing instruments,” Young says.
However, the impact of that small tweak was huge: It resulted in a slew of studies accessing ancient temperatures in a way never before possible. In one study last year, for example, Eiler and colleagues reported in Science that the fossilized teeth of Jurassic sauropods reveal a surprisingly cool body temperature of 36 to 38 degrees Celsius. “This has been a revolution,” Rumble says of the technique. And it has opened the door to new ways of fingerprinting the origins of other organic gases, he says. “This is the first conceptual breakthrough in how to go about doing it,” Sherwood Lollar says.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.