
by Adityarup "Rup" Chakravorty Tuesday, September 19, 2017
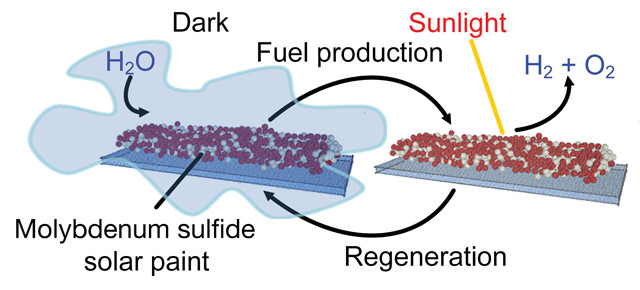
Newly developed solar paint adsorbs moisture from humid air at night and splits the water molecules into oxygen and hydrogen during the day. Credit: RMIT University.
Split a water molecule, and you get hydrogen and oxygen. Burn that hydrogen as fuel and you get water. This straightforward, pollution-free cycle is part of what makes hydrogen so tantalizing as a potential renewable fuel. Unfortunately, splitting water molecules to generate hydrogen is not currently very energy efficient. In fact, more than 95 percent of hydrogen currently used in industry is produced from fossil fuels, not water.
But, in a recent study, researchers have combined two compounds — nine parts sulfur-rich molybdenum sulfide to one part titanium dioxide — to synthesize a water-vapor adsorbing paint that improves existing water-splitting methods. Their results, published in ACS Nano, raise hope that such materials could eventually decrease reliance on carbon-based sources of hydrogen.
“The traditional approach [to generating hydrogen by splitting water molecules] has been to use purified water with specific catalysts added,” says Torben Daeneke, a research fellow at RMIT University in Melbourne, Australia, and co-author of the new study. But that requires energy for the purification steps and consumes water that could be used for other purposes. “The system we have developed is water neutral; no water purification is necessary and no drinking water is used.”
In the paint, which can be used on insulating surfaces like glass or brick walls, molecules of molybdenum sulfides form highly porous networks. The high porosity and chemical structure of these networks allow the materials to adsorb large amounts of water vapor from the atmosphere. Molybdenum sulfides are semiconductors, and pairing them with titanium dioxide — a photocatalyst that generates electric current when exposed to certain wavelengths of light — allows water molecules adsorbed by the molybdenum sulfides to be split into hydrogen and oxygen by the energy in ambient sunlight.
To test the process, the researchers placed glass chips coated with the paint in a sealed 500-milliliter glass vessel saturated with moisture. The vessels had detachable sensor chips to measure the hydrogen and oxygen produced. When illuminated, hydrogen and oxygen were generated in a 2:1 ratio, indicating that water molecules were indeed split to produce the gases.
“Traditionally, electro-catalysis [of water] has been very expensive,” says co-author Kourosh Kalantar-zadeh, a professor of engineering at RMIT. “This new process should be cheaper because molybdenum is a bulk commodity and titanium dioxide is cheap and widely used.”
How to best collect the hydrogen produced through this process remains a tricky problem that the team is working to resolve.
“There are challenges related to hydrogen storage, and the efficiency of the conversion from water to hydrogen is not yet economical,” says Brian Korgel, a professor of engineering at the University of Texas at Austin, who was not involved in the study. “I would have liked to have seen the authors calculate the quantum efficiency for the water splitting [essentially, the number of water molecules split per photon absorbed] reported in the paper.” Quantum efficiency numbers would allow researchers to determine the efficiency of this process compared to existing methods of generating hydrogen by splitting water.
Additionally, titanium dioxide only absorbs UV light, which decreases its efficiency, Korgel says. Using a material “that carries out the [photolytic] conversion using longer wavelength photons would be helpful,” he says.
In the current setup, some of the incident light doesn’t contribute to the photocatalytic process and instead contributes heat energy, Daeneke says. That heat “raises the temperature of the mixture to above 60 degrees Celsius, at which point the molybdenum sulfide can no longer hold moisture effectively.” And without moisture, there is nothing for the electric current to split, and no hydrogen production.
In their laboratory tests, the researchers found that when they coated a glass substrate with the molybdenum sulfide-titanium dioxide mixture and illuminated it, hydrogen production ceased after prolonged exposure to light. “Recharging” the paint in a dark and humid environment for 30 minutes led to almost three times more hydrogen production compared to continuous illumination.
Although difficulties remain in generating hydrogen with the new technique, Korgel thinks it’s a useful step. This is “interesting work that will probably inspire more researchers to work on the problem of photocatalytic splitting of water vapor,” he says.
Daeneke and Kalantar-zadeh next plan to test other photocatalysts that could be more efficient than titanium dioxide at absorbing energy at wavelengths throughout the light spectrum.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.