
by Justin B. Ries Thursday, January 5, 2012

Ries and one of his students collect specimens. Justin B. Ries
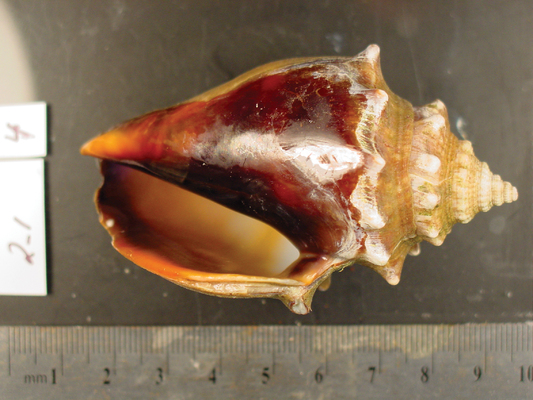
An aragonite conch shell reared under current atmospheric carbon dioxide levels (400 ppm) appears normal, but... Justin B. Ries
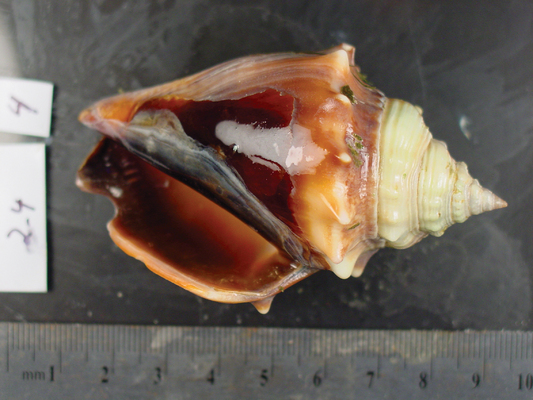
...when reared under elevated (2,850 ppm) levels, it shows clear signs of dissolution, especially at the upper lip of the shell. Justin B. Ries

An aragonite quahog shell reared at current (400 ppm) atmospheric carbon dioxide levels shows well-formed ridges. Justin B. Ries

Under elevated (2,850 ppm) carbon dioxide, however, the ridges on the shell are nearly dissolved away. Justin B. Ries

A tropical pencil urchin (high-magnesium calcite shell) reared under current (400 ppm) atmospheric carbon dioxide conditions still has well-formed spines. Justin B. Ries

When reared at elevated (2,850 ppm) carbon dioxide, however, another urchin's spines are nearly dissolved away. Justin B. Ries

Justin Ries look at 18 species of marine calcifiers, including crustaceans, corals, bivalves, gastropods, coralline algae and urchins. Justin B. Ries

Staghorn corals, such as these off of Belize, are more vulnerable to ocean acidification because they build their shells from aragonite, not calcite. Castillo, UNC-Chapel Hill
To survive in the ocean, soft-bodied organisms must possess one of five traits: big teeth, toxic flesh, invisibility, quickness or a hard shell. Most marine organisms that employ the latter, called calcifiers, build their hard shells from the mineral calcium carbonate. However, increasing atmospheric carbon dioxide levels are making the oceans more acidic — which, in turn, is reducing the concentration of carbonate ions dissolved in seawater that organisms use to build their protective shells and skeletons. Calcifying marine organisms — such as clams, crabs, corals and conchs — may soon find themselves short on building material.
However, a recent set of experiments suggests that the ocean acidification story is more complex than first thought. Whereas some marine calcifiers reared under the elevated carbon dioxide levels responded very negatively, not all of the organisms suffered in the acidified seawater. Some, in fact, appeared to benefit from it.
Carbon dioxide has fluctuated drastically throughout Earth’s past. Since the evolution of primitive marine calcifiers about 550 million years ago, atmospheric carbon dioxide is thought to have fluctuated between approximately 200 and 8,000 parts per million (ppm), with highest levels occurring in the Early to Middle Paleozoic (about 543 million to 400 million years ago), Late Triassic/Early Jurassic (about 200 million years ago), and Cretaceous (about 125 million years ago). Carbon dioxide levels dropped after the Cretaceous, however, and have not been as high as today in the past 800,000 years.
Carbon dioxide levels in our atmosphere are currently at about 385 ppm. They have been rising steadily since the late 1700s, due in large part to the burning of fossil fuels and deforestation. Modelers predict that if we don’t act to stem carbon dioxide emissions in the near future, atmospheric carbon dioxide levels could reach 750 ppm by the year 2100.
Given the extreme fluctuations in atmospheric carbon dioxide that are thought to have occurred throughout Phanerozoic time (the past 543 million years), skeptics of the ocean acidification threat often argue that many types of marine calcifiers have already survived carbon dioxide levels that dwarf those predicted for the coming centuries. Indeed, the high-carbon dioxide Cretaceous period is even named for the massive chalk formations formed from the shells of tiny calcareous plankton that flourished during this interval.
The truth of the matter is that we don’t know exactly what will happen to calcium carbonate-producing organisms when carbon dioxide levels hit certain thresholds, especially when these changes are not accompanied by the elevated seawater alkalinity that probably accompanied intervals of high atmospheric carbon dioxide in the geologic past. Thus, we set out to simulate future acidic oceans and to investigate the responses of marine calcifiers for ourselves.
We tested 18 species of marine calcifiers under four different atmospheric carbon dioxide scenarios, ranging from the current level (roughly 400 ppm) up to 2,850 ppm.
Mollusks — including oysters, quahogs, softshell clams, bay scallops and conchs — fared by far the worst under the elevated carbon dioxide scenarios. This is bad news not only for shellfish lovers, but also for the local, state and federal governments that reap substantial tax receipts from the billion-dollar industries based upon these briny delicacies.
The mollusks’ responses varied widely amongst species, however. Bay scallops, periwinkles, whelks, oysters and softshell clams built their shells more and more slowly as carbon dioxide increased. The conchs and the quahogs showed no response to carbon dioxide levels up to 900 ppm — but above 900 ppm, they showed a very negative response. One type of mollusk, the slipper limpet, showed a particularly surprising response. Its calcification rate actually increased under rising carbon dioxide levels up to 900 ppm, and only showed a decline under the highest carbon dioxide treatment (2,850 ppm). Intriguingly, the tasty blue mussel did not respond at all to the elevated carbon dioxide.
We also tested two types of urchins — tropical pencil urchins and temperate purple urchins — and two species of calcifying algae, the branching coralline red algae Neogoniolithon and the segmented calcareous green algae Halimeda. Tropical urchins showed no response between 400 and 900 ppm carbon dioxide, and then began to dissolve away rapidly at 2,850 ppm. Temperate urchins and both species of algae exhibited increased calcification up to 900 ppm carbon dioxide followed by a decline at 2,850 ppm.
Yet it was the three species of crustaceans — blue crabs, gulf shrimp and American lobsters — that exhibited the most striking response of all. They each calcified most rapidly under the highest carbon dioxide level. The response of blue crabs and the shrimp was fairly linear, with calcification rates increasing steadily with rising carbon dioxide. The lobsters showed no response to elevated carbon dioxide between 400 and 900 ppm, and exhibited an increase in calcification under the highest level.
Although not anticipated, these varied responses should not be surprising. Many of the organisms investigated in these experiments belong to groups that survived intervals of the geologic past in which atmospheric carbon dioxide was 10 to 20 times greater than that of today. Thus, these marine calcifiers must have evolved strategies for coping with such acidified conditions. And indeed, these organisms are thought to employ a wide range of relatively sophisticated mechanisms for building their shells and skeletons. So what factors are at play?
First, most calcifying marine organisms cover their shell or skeleton with some type of protective organic layer that separates them from ambient seawater. In general, organisms that produce a relatively thick organic layer that covers most or all of their shell or skeleton, such as the crustaceans, the algae and the blue mussels, were generally more resilient to elevated carbon dioxide than organisms that produce a less substantial protective barrier, such as the clams, oysters, scallops and conchs. Surely this barrier comes at a metabolic cost to the organism, but is apparently well-worth the investment when ambient conditions become too acidic.
It has been proposed that the type of calcium carbonate secreted by the organism — aragonite, low-magnesium calcite or high-magnesium calcite — should play a large role in determining an organism’s specific response to carbon dioxide-induced ocean acidification. This is because the aragonite and high-magnesium calcite forms of calcium carbonate are more soluble than the low-magnesium calcite form. But the experiments revealed that the role of mineralogy is not so straightforward. Crustaceans, for example, secrete a high-magnesium calcite shell (8 to 12 percent magnesium) but exhibited a more positive response to elevated carbon dioxide than all other investigated organisms, including those secreting low-magnesium calcite, like oysters, bay scallops and periwinkles.
Instead, it appears that the blue crab’s epicuticle layer — the outermost part of the exoskeleton — protects its relatively soluble high-magnesium calcite exoskeleton from the corrosive seawater. Shell mineralogy did appear to play a predictable role under the highest carbon dioxide level, however, as five of the six species that dissolved under these conditions formed their shells from high-magnesium calcite and/or from aragonite — forms of calcium carbonate that are less stable than calcite.
Carbonate is the most important form of dissolved inorganic carbon utilized by marine organisms in the calcification process. Yet another form of dissolved carbon — bicarbonate (carbonate plus a proton) — is 10 times more abundant than carbonate in seawater and, unlike carbonate, actually increases in seawater with rising atmospheric carbon dioxide. Organisms cannot directly use bicarbonate in calcification, but some are believed to be able to convert it to carbonate for use. Crustaceans and calcifying algae are thought to be particularly adept at this process. Thus, it is perhaps no coincidence that these organisms exhibited relatively positive calcification responses to elevated carbon dioxide.
We also investigated how photosynthesizing marine calcifiers would react to high carbon dioxide conditions. In these organisms, photosynthesis and calcification have long been viewed as complementary processes: Photosynthesis removes carbon dioxide from seawater, changing the seawater’s saturation state — less dissolved carbon dioxide means more dissolved carbonate ions. Likewise, calcification releases carbon dioxide that the organism needs for photosynthesis.
Increased dissolved carbon dioxide in seawater (resulting from increased carbon dioxide in the atmosphere) may also promote more photosynthesis, which could provide the organism with additional energy for calcification. This would result in enhanced calcification under conditions of elevated carbon dioxide despite a reduction in the calcium carbonate saturation state of the surrounding seawater.
Even though the three photosynthesizing organisms (two algae species and a coral species) we investigated exhibited either a positive or neutral response to elevations in carbon dioxide between 400 and 900 ppm, all three exhibited a substantial reduction in calcification under the highest carbon dioxide level.
This suggests that enhanced calcification due to the additional photosynthesis occurs only when carbon dioxide is the limiting factor for the rate of photosynthesis. Once there is more than enough carbon dioxide for these organisms — at around 1,000 ppm for marine algae — then the benefits of increased photosynthesis would be quickly offset by the negative effects of having fewer carbonate ions in the surrounding seawater.
Determining whether warm-water or cold-water organisms are more vulnerable to rising levels of atmospheric carbon dioxide is a pressing goal of ocean acidification research. Carbon dioxide is more soluble in cold water than in warm water — so colder waters tend to be more acidic. Organisms at higher latitudes and inhabiting deeper, colder waters are therefore expected to fare worse than organisms living in the tropical seas.
However, it seems equally plausible that the organisms living in those colder, more acidic waters would have already evolved to be more resistant to such changes in the carbonate chemistry of seawater. Indeed, the tropical urchin we tested was more negatively affected by the acidic seawater than the temperate urchin was. This may also explain why the temperate coral we looked at exhibited a more resilient response than has been previously observed for tropical corals.
These observations suggest that temperate, polar and deeper-water organisms may actually be better able to cope with the carbon dioxide-induced ocean acidification that is predicted for the coming centuries, despite the fact that colder waters will be more “acidified” than warmer waters. But much more research is needed to fully evaluate this hypothesis.
These experiments suggest that the response of calcifying marine organisms to carbon dioxide-induced ocean acidification will be variable and complex. Even organisms that calcify more quickly under elevated carbon dioxide conditions may be negatively impacted by the decline of less carbon dioxide-tolerant species within their ecosystems.
The disparate responses amongst organisms may also shift ecological relationships — particularly predator-prey dynamics — in subtle yet meaningful ways. Most of the mollusks that we looked at — the snails, clams, conchs, scallops and oysters — exhibited a very negative response to elevated carbon dioxide. But the crustaceans — the crabs, lobsters and shrimp, which typically prey upon these mollusks — exhibited a positive response. This would presumably shift this predator-prey relationship in favor of the crustaceans when carbon dioxide levels rise initially. However, over the long-term, it’s likely to mean a decline in both populations: Although crabs may initially feast more easily on thinner-shelled clams, such excesses may ultimately lead to a decline in clam populations, which could turn the crabs’ feast into famine.
One of the prevailing questions in ocean acidification research is why modern marine calcifiers would not be resilient to future increases in atmospheric carbon dioxide the way ancient calcifiers were. It’s a valid question.
However, it must first be pointed out that marine calcifiers have not demonstrated universal resilience to past carbon dioxide-induced ocean acidification events. In fact, recent work has attributed the Permian-Triassic extinction 251 million years ago — one of the most severe marine extinctions in geologic history — to a global carbon dioxide-induced ocean acidification event on the grounds that simple marine calcifiers were preferentially extinguished during this event. Multiple extinctions throughout the high-carbon dioxide Cretaceous interval have also recently been attributed to global carbon dioxide-induced ocean acidification events.
Secondly, it is not simply atmospheric carbon dioxide that determines the saturation state of seawater with respect to calcium carbonate minerals. Many factors play a role, not least of which are seawater alkalinity (a property related to the concentrations of carbonate and bicarbonate ions in seawater), temperature and the other (little-mentioned) ion involved in shell building: calcium.
Calcium is viewed as a conservative element in modern seawater: Its concentration relative to other ions dissolved in seawater remains relatively constant throughout the oceans. However, geochemical evidence suggests that the calcium concentration of seawater has fluctuated markedly throughout geologic time, in large part due to changes in the flux of brines from hydrothermal vents along mid-ocean ridges and large igneous provinces. In short, high rates of ocean crust production result in elevated concentrations of calcium in seawater.
On the other hand, ocean crust production and the resulting volcanism are also thought to be important contributors of atmospheric carbon dioxide over geologic time. Therefore, throughout Phanerozoic time, these tectonic drivers of seawater chemistry may have partially offset one another in terms of their net effect on shell-building: Increased ocean crust production would reduce the carbonate ion concentration of seawater (due to elevated atmospheric carbon dioxide), yet would simultaneously increase its calcium concentration. This suggests that marine calcifiers may not have experienced such extreme fluctuations in the calcium carbonate saturation state of seawater, after all.
Lastly, if tectonically driven fluctuations in atmospheric carbon dioxide in the geologic past occurred more gradually than the current anthropogenic rise in atmospheric carbon dioxide, then acidified seawater, as well as acidified rainwater, would have increased the dissolution of carbonates on the ocean floor and on continents. That would have increased the carbonate alkalinity of seawater, which may have rendered elevations in atmospheric carbon dioxide less harmful to marine calcifiers in the geologic past. Calcifying marine organisms also would have had more time to adapt to tectonically induced acidification that occurred over geologic timescales, rather than over the human timescales that the current anthropogenic changes are occurring.
We don’t know at what carbon dioxide level marine calcifiers — and their shells — will begin to disappear. Our experimental evidence suggests that this depends on the type of organism and its mechanism of calcification. Four of the organisms we looked at exhibited a particularly rapid decline in calcification once a critical carbon dioxide level was reached: temperate corals, pencil urchins, quahogs and bay scallops. A primary objective of future studies should be to precisely constrain these apparent tipping points. The policies and legislation that govern carbon emissions should be guided by these empirically determined thresholds.
Our inability to accurately predict how marine calcifiers will respond to carbon dioxide-induced ocean acidification ultimately stems from our relatively poor understanding of the very mechanisms by which these organisms build their shells and skeletons. Much work remains to be done on this front — and others — in order to anticipate the effects of rising atmospheric carbon dioxide and guide the policy and legislation that will hopefully curtail the looming calcification crisis.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.