
by Rachel Crowell Tuesday, October 16, 2018
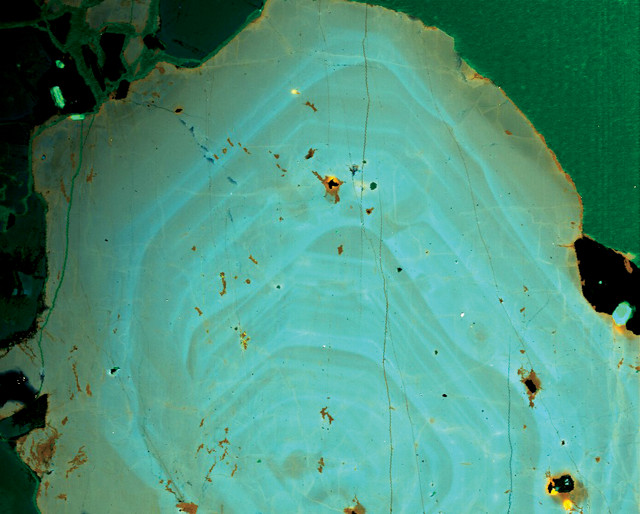
Under an electron microprobe, areas in this quartz crystal that are bluer indicate higher concentrations of titanium and higher crystallization temperatures. Credit: Michael Ackerson.
Investigating the properties of granite has given researchers insight into how gold and other economically important ores are produced, the thermal properties of Earth’s crust, the state of magma in active volcanic regions, and how Earth’s continents formed. “The temperature of granite crystallization underpins our thinking about many of these phenomena,” researchers note in a new study in Nature. “But evidence is emerging that this temperature may not be well constrained,” added the study authors, who found that certain granites crystallize at temperatures as much as 200 degrees Celsius lower than previously thought.
The conventionally accepted wet solidus for granite — “the temperature below which a water-saturated granitic melt completely solidifies” — is between 650 and 700 degrees Celsius and was established based on roughly 60-year-old laboratory experiments, says Michael Ackerson, a petrologist at Rensselaer Polytechnic Institute in New York, and lead author of the study. In those experiments, researchers heated granite powders until they melted, and these melting temperatures were then used to determine the granite solidus, Ackerson notes. Although these early results have been repeated, they may not accurately represent the temperatures at which granite solidifies in nature, he says.
His team combined two independent techniques — titanium-in-quartz thermobarometry and diffusion modeling of titanium in quartz — to evaluate the crystallization temperatures of rocks from the Tuolumne Intrusive Suite (TIS), a collection of granites found in the Sierra Nevada of Yosemite National Park. They chose to study quartz because it’s “one of the most abundant minerals in granitic rocks, which means our approach could potentially be applied to granites across the globe,” Ackerson says.
The TIS, which includes the Cathedral Peak granite and the Half Dome granodiorite, is the youngest intrusive formation among the seven found in Yosemite. It is also the most extensive bedrock formation in Yosemite, covering about one-third of the park’s area. Samples from the TIS were chosen because they are easy to access, relatively fresh and pristine, Ackerson says.
He and his colleagues found that their quartz samples crystallized at temperatures ranging from 474 to 561 degrees Celsius.
For the thermobarometry technique, the researchers used an electron microscope to quantify the amount of titanium in each sample, which depends on the temperature and pressure at which they crystallized, Ackerson says. Higher titanium concentrations indicate higher temperatures or pressures at the time of crystallization.
After assuming that the rims of these quartz crystals “represent late-stage, near-solidus crystallization,” the researchers linked the granodiorite wet solidus with a model for titanium concentration in quartz. That model describes the titanium concentration in quartz as a function that varies with different values for temperature, pressure, and titanium concentration relative to saturation of rutile — the most common natural mineral composed primarily of titanium dioxide — in the melt.
The researchers found that if crystallization occurred at pressures between 1.6 x 108 pascals and 2.4 x 108 pascals — the range found roughly 6 kilometers below Earth’s surface — the crystals’ rims would have titanium contents ranging from 132 to 219 parts per million (ppm), Ackerson notes. Those pressures are required for the quartz to crystallize at the wet solidus of granite. However, the researchers observed titanium concentrations of 20 ppm to 40 ppm, suggesting that the quartz crystallized at lower pressures. Thus, “the main volume of quartz within these granitic rocks crystallized below the expected wet solidus temperatures,” Ackerson and the other researchers wrote.
“The arguments in this paper are very sound,” says Craig Lundstrom, a petrologist and geochemist at the University of Illinois at Urbana-Champaign who was not involved with the study but has extensively studied granite formation. “There is controversy surrounding the use of the titanium-in-quartz thermometer, and some might question the conclusions based on that alone,” he adds, but “what makes the work powerful is that the authors use a second method involving modeling of diffusion profiles that gives the same low-temperature requirement that the thermometer does.”

Matterhorn Peak in the Cathedral Peak Granodiorite is part of the Tuolumne Intrusive Suite, a collection of granites from the Sierra Nevada. Researchers studied quartz in this granite to determine granite crystallization temperatures. Credit: Dug Ross, CC BY-N.C 2.0.
To model titanium diffusion, the team used an electron microprobe to map titanium concentrations across cross sections of quartz crystals from the TIS, and then compared those maps to computer models of how titanium concentrations evolve in growing crystals. The microprobe images look something like cross sections of trees with numerous growth rings, Ackerson says. “We should see a lot more blurring of these growth rings” if the quartz was exposed to higher temperatures for long periods of time, as would have happened if this granite crystallized at 650 to 700 degrees Celsius, he says. Instead, there are regions with sharp distinctions between adjacent rings, which show “where there was an abrupt change in how that quartz was crystallizing.” This pattern “can only happen if [granite] crystallizes at really low temperatures,” he adds.
Lundstrom co-authored a 2009 study describing laboratory experiments that showed that “a granite could form at 400 degrees [Celsius] when intermediate-composition hydrous magma was placed into a temperature gradient for two months,” Lundstrom notes. “A 2-centimeter-long capsule with andesite and 4 percent water was placed into a temperature gradient that spanned 950 degrees Celsius down to 350 degrees Celsius. Over the two months, there was a continuous reaction and diffusive transport that resulted in a gabbro forming at a higher temperature and granite at a lower temperature,” he says.
The work of Ackerson’s team “perhaps provides evidence that processes like those in the laboratory may actually occur in making granitic intrusions — and thus even in making continental crust,” Lundstrom says.
The results of the study have diverse implications, Ackerson says. For instance, while “certain deposits that form gold, copper and molybdenum occur directly above granites — hence their location is already known — there is some debate about how these types of important ore deposits form,” he says. “Part of the conundrum,” he adds, is “in understanding the compositions and temperatures of the fluids that generate the ore deposits. For example, are the fluids that crystallize these ore deposits derived from the granites, or do the granites merely supply heat to the overlying rock to form the ore deposits?” And “if granites crystallize at lower temperatures, does this also mean that these ore deposits can form at lower temperatures as well?”
Moreover, lower granite crystallization temperatures may impact researchers’ understanding of how Earth’s continents, formed primarily of granite, took shape, Ackerson notes. “If we didn’t have granites, we wouldn’t have continents like we know them today.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.