
by Lisa Song Thursday, January 5, 2012
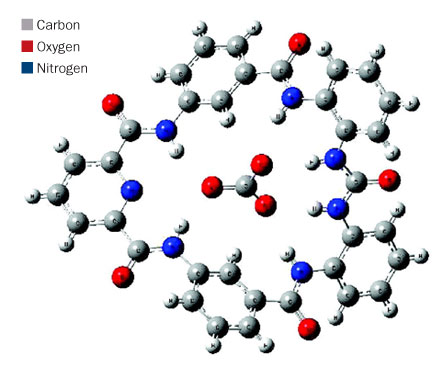
The shallow bowl-shaped molecule shown here can trap an ion of carbonate (at center). Once the carbon dioxide is caught, heating the entire complex frees the gas so that it can be stored underground. J. Tossell
Thanks to a bit of luck, the key to carbon sequestration may lie in a circular, bowl-shaped compound that draws carbon dioxide right out of the air.
Researchers at the University of Maryland in College Park had been working on a compound synthesized for biochemical applications. Upon closer inspection of the compound, they made a surprising discovery: It sucked carbon dioxide out of the lab’s air. The results caught the attention of Jack Tossell, a chemistry professor at George Washington University in Washington, D.C. Although Tossell was not involved in the original biochemical research, he began studying the compound’s properties after learning of its potential for carbon storage. “The point [of my research] is to encourage people who are doing chemistry to think about the applications of their work to global climate change,” he says.
In carbon sequestration, carbon dioxide is usually captured from smokestacks, then stored underground in geologic formations that prevent the carbon from escaping into the atmosphere. Some power plants use ammonia to scrub carbon dioxide from their flues, but this new compound is one of the few technologies that trap carbon dioxide directly from the air, Tossell says.
The reaction results from a kind of chemical soup: Scientists mix various biochemicals (such as ureas and pyridines) and apply heat. When carbon dioxide in the air reacts with water in the solution, carbonate forms. The final compound, known as a complex, is shaped like a shallow bowl with a carbonate molecule trapped in the center. Once the carbon dioxide is caught, heating the entire complex frees the gas, allowing it to be stored underground, Tossell suggests in the journal Inorganic Chemistry. Meanwhile, the rest of the complex can be reused to trap more carbon dioxide.
One advantage of the complex is its stability — it takes little energy to draw carbon dioxide from the air, making the complex very efficient, Tossell says. But consequently, more energy is then needed to release the carbon dioxide for eventual storage. “It’s always this competition,” Tossell says. “You want a complex to be stable enough to pull down the carbon dioxide but then you want to get the carbon dioxide back out by breaking the complex.”
There is much to be done before the technology can be scaled up for commercial use, Tossell noted in the paper. For example, the complex requires dozens of atoms to keep one molecule of carbon dioxide in place, so manufacturing large amounts of the complex is simply infeasible. “We need something simpler,” Tossell says — and he and his colleagues are working to develop a version of the complex with fewer atoms.
Ultimately, Tossell says, the complex provides a good starting point, and opens doors for inorganic chemists. “My research paper is a conventional computational chemistry paper, but with the suggestion that we should think about possible uses of computational chemistry to fight climate change.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.