
by U.S. Geological Survey Wednesday, June 13, 2018
James F. Carlin, Jr., a mineral commodity specialist for the U.S. Geological Survey, compiled the following information about bismuth, an essential commodity for the cosmetic, foundry, pharmaceutical, aluminum and steel industries.
Bismuth is a brittle, silvery-white metal with a low melting point and a high density approaching that of lead. Alloys of the metal with lead and tin are known to have been used since the Middle Ages. The metal was referred to as wismuth, and at the end of the 16th century, Georgus Agricola, an early mineralogist, Latinized the Germanic name to bisemutum.
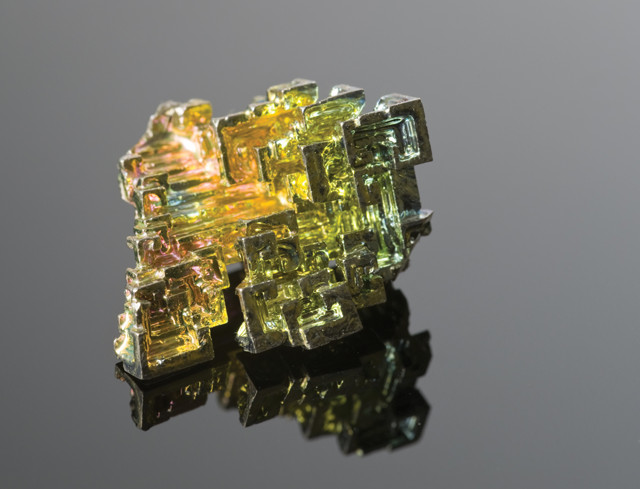
Free bismuth oxidizes easily, producing an iridescent surface. Credit: ©Shutterstock.com/MarcelClemens
Bismuth compounds were initially noted for their soothing effects on wounds and sores. Around 1597, the efficacy of bismuth nitrate for treating intestinal disorders was discovered, and it is still used today in stomach ailment remedies. These properties were also valued in many medicinal and cosmetic preparations, which until 1930 accounted for about 90 percent of the bismuth used. The largest single use of bismuth continues to be in the pharmaceutical field.
The average bismuth content of Earth’s crust is estimated at 0.1 parts per million (ppm), and it rarely occurs in sufficient concentrations to permit commercial recovery as a primary product. It is typically recovered as a byproduct during the processing of ores of other metals, principally lead. Therefore, the supply of bismuth is dependent on demand for these metals.
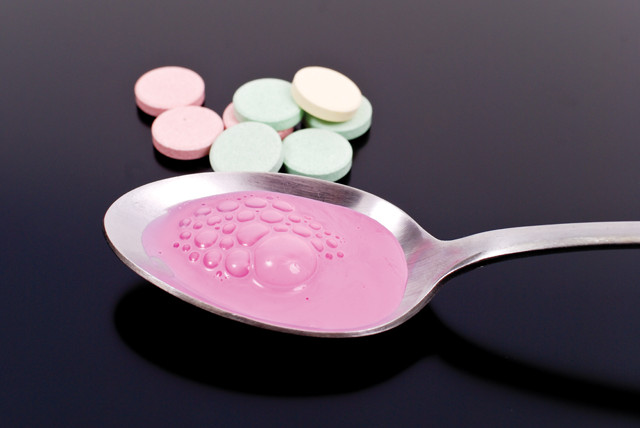
The active ingredient in the stomach ailment remedy Pepto-Bismol® is bismuth subsalicylate, which acts as an antacid, anti-inflammatory and antimicrobial agent. Credit: ©Shutterstock.com/jcjgphotography
Bismuth was initially produced by smelting bismuth-rich ores to produce crude metallic bismuth. In the 18th century, pure bismuth metal was produced, demonstrating that it was a distinct element, with properties similar to those of lead.
The association of bismuth with base metal ores and the fact that it accompanies lead through smelting and refining operations allow for economic extraction of crude bismuth at base metals refineries. Continued improvement of metallurgical processes for extraction and purification of bismuth have resulted in the production of commercially available bismuth metal of 99.995 percent purity.
The subsequent development of low-melting-point alloys and chemical catalysts containing bismuth, its use as an additive to casting alloys, and other more limited specialized applications, along with applications in free-machining steel and aluminum and malleable iron castings, have resulted in a variety of industrial applications for bismuth.
For more information on bismuth and other mineral resources, visit http://minerals.usgs.gov/minerals.
Production and consumption
World bismuth mine production in 2012 was estimated to be 7,400 metric tons, 81 percent of which was mined in China.
Although there is no bismuth mining in the U.S., a small amount of bismuth is produced from secondary (scrap) sources.
In 2012, U.S. apparent consumption of bismuth was estimated at 1,000 metric tons, most of which was imported from China.
Bismuth is relatively nontoxic, allowing it to replace lead in many applications, such as birdshot, lubricating greases, pigments and solder.
Fun facts
Alloys of bismuth with tin, lead or other low-melting-point metals are used as triggers on fire sprinkler heads.
Bismuth is one of the few metals that expands as it solidifies, and thus is used in a variety of applications that require a tight grip, such as devices to hold optical lenses during grinding.
Bismuth’s natural abundance is highest in oceanic manganese nodules, where it ranges from 0.5 to 24 ppm. It is next highest in silicic rocks, where it ranges from 0.02 to 0.9 ppm. Its abundance in coal ash may be as high as 30 ppm.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.