
by Carolyn Gramling Wednesday, May 23, 2018

Peter Brewer. Credit: Todd Walsh © 2004 MBAR
Whether through deep-sea gas hydrate experiments, lasers or asphyxiating squid, Peter Brewer is always seeking new ways to understand the changing chemistry of the oceans. Brewer has embarked on a series of groundbreaking projects throughout his career. During his nearly 24 years at the Woods Hole Oceanographic Institution, he was the chief architect of the Joint Global Ocean Flux Study, launched by the United States in the late 1980s to track the cycling of carbon and other chemical elements between the atmosphere, oceans and seafloor — and to understand human impacts on these cycles.
In 1991, Brewer became president of the then-fledgling Monterey Bay Aquarium Research Institute (MBARI). Over the next six years, Brewer helped turn MBARI into a new kind of oceanographic institution, as envisioned by its founder, David Packard (of Hewlett-Packard): a facility that would combine cutting-edge technology with scientific curiosity to penetrate the deepest, darkest reaches of the ocean. And in 2007, he won a Nobel Prize as a member of the Intergovernmental Panel on Climate Change.
Now a senior scientist at MBARI, Brewer is still at the forefront of ongoing scientific research and discussion, investigating storing carbon dioxide in the seafloor, the fate of World War II-era chemical weapons in the ocean, and ocean acidification, which he calls the “bitter twin” of global warming. He recently spoke with EARTH reporter and Web editor Carolyn Gramling about his long, varied career.
CG: You started out as a chemistry major in college at Liverpool University in England. How did you switch to oceanography?
PB: The way they were teaching chemistry seemed to me to be pretty grim — the future of making polymers, things like that. I had heard rumors that there was a need for chemists to go on a trip to the Indian Ocean and that sounded like fun.
Technically, my first cruise was a training trip on the Irish Sea measuring current velocities. We’d do day trips, get drunk on weekends. But my first big trip was that international Indian Ocean expedition from 1963 to 1964. I was a graduate student by then — we were basically slave labor, out for nine months at a time: 30 days at sea, four days in port. I got to see national parks in Africa, climb hills in India, explore the world, and along the way rich and famous scientists would fly in and I’d make friends with them. So I rapidly became known to a large number of big names in the field, and I learned from them.
CG: What sorts of things did you study on that Indian Ocean trip?
PB: At the bottom of the Red Sea, we found the world’s first hot vents. At the time they weren’t called hydrothermal vents; they were called hot brines. But it’s the same geochemistry — in the Red Sea, they erupt through a massive layer of basalt. Instead of coming out with abundant life, it’s hot water with huge amounts of sodium chloride that form these pools of water in which life can’t survive. But if I were to do a chemical analysis to remove the sodium chloride, the fluid would be identical to [hydrothermal vents].
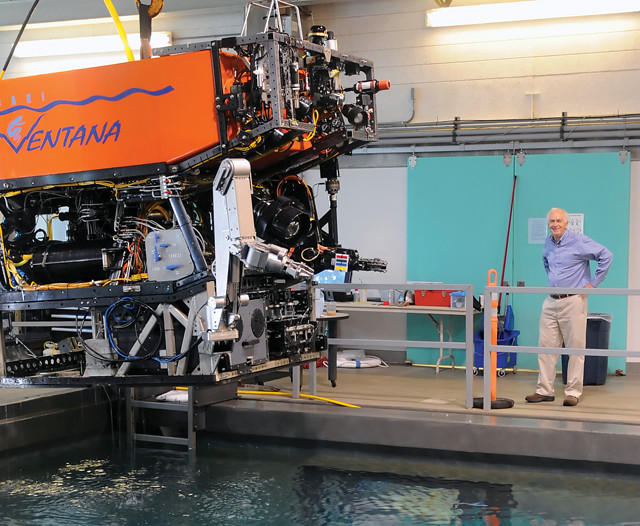
The ROV Ventana lowers into MBARI's test tank in preparation for a test to set up Brewer's seafloor carbon dioxide experiments. Credit: odd Walsh © 2008 MBARI
CG: Was that your thesis work?
PB: I did a bunch of work for my thesis on various things, but finding those hot brines in the Red Sea was of remarkable interest. I was just a student, so I did all of my own analyses. But the Woods Hole Oceanographic Institution (WHOI) had also sent a ship out there, and they took some samples too. They put a team of 20 people on it. And that turned out to be a problem. When the results were published, they realized they had all done the work individually, and the left-hand side didn’t balance the right-hand side.
The good thing was that John Hunt [then-chair of the Department of Chemistry at WHOI] looked at my work and realized that a single young person on a small bench had aced the problem — and I was offered a job.

In an unusual and groundbreaking experiment, Brewer's team put carbon dioxide into a glass beaker on the seafloor to test whether the greenhouse gas could be stored at the bottom of the sea as gas hydrate. The result was striking and unexpected: Instead of solid hydrate, jelly-like blobs formed as a result of the carbon dioxide-seawater reaction. Credit: 1998 MBARI
CG: After several decades at WHOI, you left to become head of MBARI in 1991. What drew you there?
PB: MBARI was begun in a small way in 1987 by David Packard. Dave wanted a research institute that would match the kind of technical advances he saw during his term as deputy secretary of defense (1969 to 1971). He wanted working tools: ROVs [remotely operated vehicles], undersea instruments.
[With earlier directors,] it didn’t go well. One director was Dick Barber — he wanted to do a classical ocean science thing to measure the ocean surface, chlorophyll. But Dave wanted engineers to build undersea vehicles. There was turmoil, and I was brought in. I accepted the job from the emergency room at Falmouth Hospital [in Massachusetts]. My daughter had broken her arm rollerblading.
CG: Did you know what you were getting into?
PB: Not really. At the time, the institute was on a rocky footing socially. Scientists would come in from a traditional science background and engineers from industry, and they expected to do what they were used to. People were not quite sure what to do. It took a little time to overcome that.
But Dave was great; he had this tremendous vision. We built an institute with a strong focus on the deep ocean as opposed to the surface. That was what he wanted — [he believed] any institute could focus on the surface. He wanted advanced tools for undersea research.
CG: You have a number of interesting research videos on MBARI’s Web site — I’m thinking of the beaker experiments where you inject carbon dioxide into seawater at ocean pressures and it forms these jelly-like blobs.
PB: The background for that is that for 25 years, there had been suggestions that we should dispose of carbon dioxide in oceans. There was a government program, chaired by the now-presidential science advisor John Holdren, which came out around 1996 that took a look at the nation’s energy future and the carbon crisis. Two of the things they were interested in were methane hydrates as a future energy source and disposing of carbon dioxide in the ocean by making it form hydrates — you would inject it into the oceans where it would form a stable white crystalline solid, sit there and pile up.
I didn’t think that was the way it would work chemically, but we decided to try some deep experiments with beakers. So in January of 1996, we began our first experiments using our vehicles to carry out controlled chemical experiments. I went out to sea where we did an experiment on methane. It was a very simple experiment: We injected methane gas [at the ocean bottom] and showed we could make massive amounts of hydrate in minutes. They behaved very strangely — odd blobs appeared. It’s been hard to reproduce that. Sometimes it happens and sometimes it doesn’t, and we’re not sure why. But just showing that video answered a lot of questions we’d had from lab experiments. And it was the first time that our undersea vehicles had been used not for collecting animals or rocks — it was a better use of our vehicles and allowed us to use the local waters as our laboratory.

Brewer holds his 2007 Nobel Peace Prize, which he received as a member of the Intergovernmental Panel on Climate Change (IPCC). IPCC shared the award with former Vice President Al Gore. Credit: Todd Walsh © 2008 MBARI
CG: At the American Geophysical Union’s meeting last fall, you gave a talk about the effects of ocean acidification on sea creatures, which included another fascinating video of its effects on a squid in a test chamber. We actually see the squid reach its carbon dioxide threshold and flatline.
PB: Yes, that was quite striking, the squid video. Equations mean one thing, but a visual image means another. We are trying to define on some practical basis the limits of life in the ocean, thermodynamically. How does an animal feel the difference? Is it more work to swim? What does it sense — 1 percent, 10 percent difference [in ocean acidity]? Where’s the cutoff? There are a lot of fuzzy numbers in the literature.
If you think of human beings, we can survive, but we can’t sustain a society above 12,000 feet. A woman can’t carry a child to term [at that altitude]; there’s not enough oxygen to the placenta. It’s like that with the ocean. What will happen in practice is that animals will simply start to avoid the regions that are chemically stressed. You will start to get large empty zones. Fish can swim through the zone, but they wouldn’t want to linger. Other animals would simply avoid the space.
CG: You’ve also worked on identifying the location of chemical weapons dumped in the ocean after World War II. That seems like a bit of a departure. How’d you get involved with that?
PB: I was on a government committee that tackled that issue. Technically, it’s a question of applied physical chemistry. I’m at an ocean science institute; every morning, I see these standard navigational charts when I go to get coffee. For 75 miles from my office, there’s a large block of the ocean floor marked “chemical weapons disposal.” There are seven blocks [in the Pacific] marked chemical weapons disposal. I began to ask what was out there.
Basically, there’s just a lot of confusion. It’s been kind of swept under a rug. The Navy probably put stuff down there; the Army officially reports on chemical weapons issues, but it doesn’t really want to know anything about the bottom of the ocean; and NOAA doesn’t know. The Army reports one site off California, but the charts say seven sites. Which is it? How did those sites get on the charts? Nobody knows.
The situation’s even murkier in Japan — we know there are a large number of sites off Japan, but U.S. charts don’t report any of them. From a geochemist’s point of view, the chance of hitting something on the seafloor randomly is very small. But on the other hand, it’s smarter to know a bit more about these things.

Brewer has experimented with how an increasingly acidic ocean affects its denizens, such as squid. Credit: © iStockphoto.com/Heike Loos
CG: You’ve worn a lot of different hats over the course of your career. What’s the link between all the different things you’ve done?
PB: I’ve been measuring ocean carbon chemistry in some form or another for a long time. I made my first measurement of carbon dioxide in the ocean in 1965. It’s been a nice niche to pick apart the strange behavior of carbon dioxide and how it works, and realize its impact.
It’s hard for many people to find a way to link the kind of science they get in textbooks with the wonderful experience of observing something as massive and strange as the ocean. We’re trying to find ways to do that. This business of making a match between fundamental techniques in the world of chemistry and applying them to measuring Mother Nature in a very direct way — it’s a nice niche to be in.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.