
by Carolyn Gramling Tuesday, January 2, 2018

Kateryna Klochko headshot. Credit: Carnegie Institution of Washington.
Kateryna Klochko made a splash in the ocean geochemistry world as a doctoral student at the University of Maryland in College Park. She had been in the U.S. for only a few years, following a long journey from her native Ukraine. Yet she was about to challenge paradigms.
Klochko was interested in climate and geochemical cycles in both ancient and modern oceans. Boron isotopes in marine carbonates have been considered to be a good proxy for the acidity of ancient oceans — and thus for the carbon dioxide content of the ancient atmosphere.
But some scientists questioned the validity of this proxy — and she found those questions interesting, Klochko says. “There were a number of assumptions” built into the proxy, she says. “There was not enough fundamental knowledge about it.” So for her dissertation, Klochko set about systematically re-evaluating what was already known, and what was assumed, about boron geochemistry.
Using boron isotopes to estimate pH requires understanding which boron molecules would be present in the water at different acidities, and exactly how those molecules would interact with the rocks. Klochko’s re-examination of pH reconstructions based on boron geochemistry stirred a lot of controversy — but has also fundamentally altered how scientists think about reconstructing ancient ocean pH from carbonate rocks. That courage to question an established paradigm earned Klochko an Outstanding Women in Science Award from the Geological Society of America in 2010.
Klochko is now a postdoctoral research associate at the Carnegie Institution of Washington in Washington, D.C., working on how chemical molecules in solution interact with the surfaces of solids, such as carbonates. Recently, Klochko talked with EARTH’s Carolyn Gramling about her work.
CG: How did you become a geologist?
KK: I grew up near Dnepropetrovsk State University in Ukraine. I wasn’t particularly interested in science, but I grew up in a very educated family. My mom was a mathematician, and the first of her family to go to university. My father died when I was 10, and my mom had to work a lot, so I spent a lot of time with the neighbors across the hall. I would go over there to dinner, and the husband, a chemist, would ask me questions while we ate: If a train is going this way, and a bullet is flying at this speed. …
My mom wanted me to study applied math at university, but to go to university, you have to prepare yourself beyond the high school level — there is a big gap between the high school level of education and the expectation at university. You have to decide on your department right away, and take classes only in that department. I think my preparation was insufficient for applied math, but I got in the engineering geology department. I do not regret a single day how that turned out. I love Earth and I love nature.
In the former Soviet Union, geologists were considered the elite of science — they were explorers, risk takers and people with a great “generosity of spirit.” There was normally not much money to support the work, and they had to risk their lives. In books we read as children, geology was a very romanticized science — I imagined geologists as bearded, rugged guys with a hammer.
But I learned there are so many other aspects of geology as well — geobiology, organic chemistry. And we had field trips — to Crimea, to the mountains — it was wonderful.
CG: Why were you interested in studying boron?
KK: I’m interested in climate, and when I started my Ph.D. at the University of Maryland in 2003, I knew I wanted to do something with environmental climate and climate change. [Geology professor] Jay Kaufman said he had an interesting project. He wanted me to work on a technique to measure boron isotopes in ancient carbonates.
At that time, boron [in marine biogenic carbonates] was established as a pH proxy in the oceans. When I started out, I wanted to use that proxy just like everyone else.
It works like this: There are two species of borate in seawater, and the distribution of those species is pH-dependent. That’s the key. If one of those two species gets incorporated into a marine carbonate, that carbonate carries the isotopic signature of that species. And if you measure that signature very precisely and accurately, and know what species you are looking at, you can calculate the pH, ideally. The carbonates that are normally used in studies like these are corals and foraminifera. Those are living organisms that utilize the elements provided by the oceans to make their living and in the process they build their skeletons in very elaborate structures composed of calcite or aragonite.
I started working with Carnegie Institution of Washington geochemists Steven Shirey and Tim Mock on developing a laser ablation technique to measure boron isotopes in carbonates. But the more I read about it and talked to people, the more I thought it might be better to look at the proxy itself first.
CG: What were some of the questions about the proxy you wanted to investigate?
KK: There is an isotopic fractionation between those two borate aqueous species. The constant that people used to reconstruct ancient pH came from 1977; it was calculated based on thermodynamics.
Then, in 2000, pioneering scientists from Columbia University’s Lamont-Doherty Earth Observatory produced a very important dataset. They cultured several species of biogenic carbonates and grew inorganic calcite in controlled pH conditions. This important calibration has shown that there is indeed a strong relationship between pH and the boron isotopic composition of these carbonates.
There was one problem, however. Their data fell outside of the range of possible boron isotopic compositions offered by the 1977 fractionation constant. Plus, there were offsets between the datasets. And nobody could explain why. There are no known processes that would cause that offset — so that tells us something else was going on: Either the datasets were off or the constant was in fact larger than we formerly thought.
And that was when we realized that the fractionation constant wasn’t ever [directly] measured. It seems to me so fundamental: You need to know everything you can about that system.
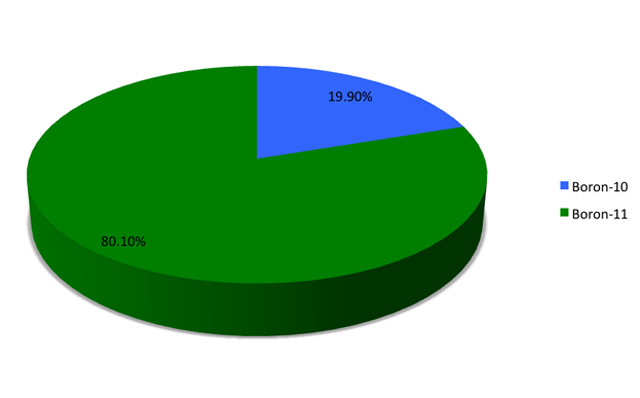
A chart showing the abundances of the naturally-occurring isotopes of Boron. A chart showing the abundances of the naturally-occurring isotopes of Boron. Credit: Jacob_S-589 (CC BY 3.0).
CG: So you decided to measure the constant?
KK: Yes. But it’s difficult. I met [Maryland] chemistry professor Jack Tossell, who was working on the calculations on the fractionation constant using molecular orbital theory, and had come up with a different constant. We were trying to find a way to directly measure the fractionation constant between the two species [each one separately] in seawater. We didn’t know how to approach this … because in solution, you have both forms of boron, borate and boric acid, together. So we were thinking about it all wrong.
Then [Tossell] remembered he knew someone at the University of South Florida who had done some work on borates in seawater earlier in his career: Robert Byrne. And Bob said that he might have an idea how to measure this, but he needed some time.
One day I was driving on Interstate 495 when Bob called me. I hadn’t met him yet, only had some phone conversations. And when he called me, of course I have to talk to him — I didn’t feel like I could tell him, “You know what, I’ll call you back.” So I pull over onto the shoulder, and he says, “Katya, I know how to measure the constant! You have to get down here!”
So I’m sitting and talking to him and somebody knocks on the window. It was a state trooper. [Byrne] is explaining the method to me at this point and I don’t want to miss it, so I’m writing things down. I roll down the window and tell the cop, “Can you wait a minute?” He’s saying, “Ma’am, you have to move.” So I would start moving a little bit, while also writing.
CG: Did he give you a ticket?
KK: No! [Laughs] So I went [to Byrne’s lab] between classes and teaching assignments, and worked on the measurements [of the constant]. We received absolutely no financial support to do this work — even though we applied three times. That’s why getting this done was especially difficult. But we managed; Jack Tossell ended up spending almost all his own grant support.
CG: So why were there offsets?
KK: The fractionation I measured was indeed larger — 27.2 parts per thousand — than the one commonly applied, 19.4 parts per thousand. That finally made more sense out of the calibration data.
But then there was another proxy condition I wanted to check: How well do we know which borate species are directly incorporated into the carbonate? Turned out it was not so well. I turned to George Cody, a staff scientist and [nuclear magnetic resonance] expert at Carnegie Institution of Washington. After analyzing borate coordination in several corals and forams, George found both borate species — but the isotopic composition did not correspond to this inventory. With George, we are working on a series of experiments that will help us look at the structure of borates on the surface before they get locked in the carbonate lattice.
The question of the offsets remains. Geochemists treat isotopic and elemental composition of abiogenic carbonates as a product of so many factors: vital effects, inorganic effects, analytical artifacts. I think in the case of the boron isotope system, it is a combination of all of those things.
CG: So how has all of this been received by your colleagues in the field?
KK: I had read all these papers by the Lamont people on this topic. And of course, they were my gods. When I presented at [a meeting of the American Geophysical Union], I was so excited; we measured the fractionation constant!
Some scientists were very encouraging — saying “finally someone’s doing something about the proxy.” Others insisted that the [calculated] slope from the Lamont carbonate calibration datasets was sufficient for paleo-pH reconstructions.
After I defended [my dissertation] last year, I was invited to Lamont to give a talk and participate in a debate/discussion guided by Wallace Broecker, with the leading scientists in the boron community. We presented our work and discussed the issues of the proxy. I was afraid how they would perceive what I’d done. I was really intimidated. I was so concerned that I did not sleep for weeks prior to that.
But it was an excellent meeting. For me, the highlight of this was being able to present our work to Wallace Broecker. He was so supportive and encouraging! It gave me motivation to work harder on this. That was my validation that I’m on the right path.
I believe that with any proxy with any system you’re studying, in order to apply it, you need to understand the mechanisms behind it. For me, it is the key; you can’t just look at the isotopic composition or the slope of your calibrated curve. It’s not enough; you need to try to understand all the mechanisms, as far as your resources can take you. We cannot do everything, but we can do more. We can go farther and study more. So I’m still working to understand what happens to a borate molecule on its way from the aqueous environment to its final position in the carbonate lattice. That’s what I want to understand!
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.