
by Sam Lemonick Tuesday, January 27, 2015

Deanna D'Alessandro. Credit: Sam D'Agostino, SDP Photo.
Deanna D’Alessandro, a chemist at the University of Sydney in Australia, is working on a new material that may make it cheaper and easier to clean greenhouse gases from power plant emissions. D’Alessandro has developed crystals composed of metals and organic molecules that can trap molecules of greenhouse gases like carbon dioxide, and release them on cue — or even transform them into more useful compounds. Her work won her one of three L’Oreal Australia For Women in Science Fellowships given last year.
D’Alessandro, who did her undergraduate and doctoral work at James Cook University in Queensland, Australia, will get more than $6 million over the next five years from the Science and Industry Endowment Fund in Australia for collaborative work with Cameron Kepert, another University of Sydney chemist further exploring the potential these materials hold. She talked with EARTH contributing writer Sam Lemonick about how her new materials could solve problems for industry and the environment.
SL: You’ve attracted a lot of attention recently for new materials that could reduce greenhouse gas emissions from power plants. Tell me more about the materials.
DD: Our work involves looking at how we can capture greenhouse gases like carbon dioxide and methane using porous materials called metal-organic frameworks. They’re a little bit like crystalline sponges made up of metals linked by carbon and nitrogen. There’s a lot of porous space within them so carbon dioxide can be preferentially adsorbed over other gases.
SL: How are the greenhouse gases trapped?
DD: The molecules could attach to the metal or to groups on the [carbon and nitrogen] linkers. We’ve incorporated amines [molecules with a nitrogen bonded to three other atoms] into these materials, which are the basis of the industrial process for carbon dioxide capture.
SL: What process is used at power plants today?
DD: [Power plants] currently use amine solutions that react preferentially with carbon dioxide. They’ve had a lot of problems with the procedure and that’s the reason why it hasn’t been rolled out in power plants across the world. It’s very expensive and it can take up to about 40 percent of the energy of a power plant just to capture the carbon dioxide using this very old method, which was patented back in the 1930s. That’s the reason why there’s a big push toward materials that are much more efficient at doing that capture.
We can tailor the materials in a number of different ways. We can put amines on the metal centers, we can put amines on the linkers, and we can incorporate other groups into these materials that have a particular affinity for carbon dioxide. We can really fine-tune the amount of a greenhouse gas that’s taken up but we can also tailor the gases that are being separated quite well.
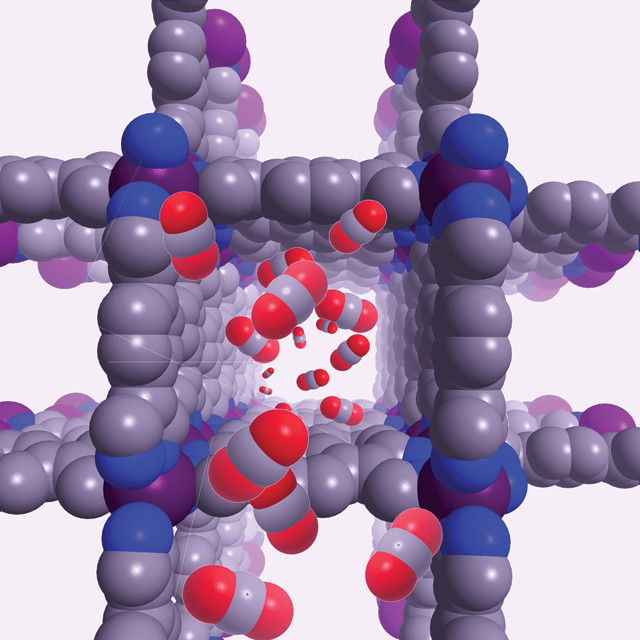
An illustration of D'Alessandro's carbon dioxide-trapping crystal lattice. Credit: Deanna D'Alessandro.
SL: How would these metal-organic frameworks be applied in, say, a coal plant?
DD: The current method uses a big adsorption tower of amine solution. The power plant’s flue gas bubbles through it. The carbon dioxide reacts with the amine, and the solution is passed off to a desorption tower where it’s heated up to get the carbon dioxide off [the amine].
[Our process uses] solid materials; we see these as having an advantage over the expensive solution-based processes. The energy savings comes in the fact that we use these in a membrane-type process, so it works on a pressure differential across a membrane made of these materials. That has massive cost [and energy] savings over a temperature-based swing [process] that is currently used.
The question is always, “What do you do with the carbon dioxide you capture?” A lot of people ask why we don’t use the material to capture the carbon dioxide and then store it or bury it or something. In the U.S., they traditionally compress the carbon dioxide and try to store it somewhere. In fact, there’s a better cost saving if we use it as a regenerable material rather than just storing it. We’re thinking about ways to transform that carbon dioxide once we’ve actually captured it. The nice thing is that these materials can do that as well.
SL: What could you do with the carbon dioxide your materials trap?
DD: A very current research project is transforming that captured carbon dioxide into another product, like methanol or oxalic acid. There are a number of feedstocks [compounds commonly used to synthesize other products] that could be made from carbon dioxide.
Unfortunately, carbon dioxide is a stable molecule so we need to put quite a lot of energy into it to transform it into anything. We’re working on ways to use light to [catalyze] that process.
SL: Last year you were named a L’Oreal Australia For Women in Science Fellow. That must have been a big moment in your young career.
DD: It was a great thing because I’m just starting my own research group. That little bit of money and that little bit of recognition was a stepping-stone. I don’t like awards for the sake of awards but it has allowed me to do other things scientifically that have been very important in getting my research career off the ground. [The L’Oreal Fellowship] was a precursor to a larger grant. We now have a consortium of people working on the carbon dioxide project.
SL: What is your next research project?
DD: Right now I’m just trying to keep my head above water!
Longer term, the reason I got into chemistry was to work on problems that were important for the world. I’ll probably continue to work on problems related to environmental issues.
I have other projects related to electron transport in natural systems. There’s a big interest in making lightweight conductors, so we’re working toward making conducting porous materials [for use in] batteries, cathodes and so forth. Very lightweight materials would significantly decrease the weight of batteries. That’s quite a big issue in space applications, for example.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.