
by Carolyn Gramling Thursday, January 5, 2012
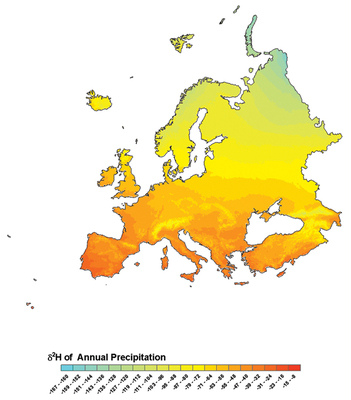
This isoscape - or isotope map - shows the distribution of the stable hydrogen isotope deuterium in precipitation across Europe. If researchers know what the correlation is between the deuterium isotopic values of human hair and the deuterium isotopic values of source water, in conjunction with a sequential analysis informed by the hair's growth rate, they can roughly trace a person's recent geographic trajectory. Gabe Bowen, Purdue University

Researchers at the Stable Isotope Forensic Laboratory examined strands of a John Doe's hair to determine where he had spent the last few months of his life to help identify him and solve his murder. Kemp and Meier-Augenstein

Helen Kemp, Wolfram Meier-Augenstein (pictured below) and other researchers at the Stable Isotope Forensic Laboratory at the James Hutton Institute in Scotland work to create isoscapes: maps that show spatial distributions of stable isotope ratios in different parts of the world. Kemp and Meier-Augenstein
In November 2006, someone left a badly beaten man at a hospital in Gwent, South Wales. The victim was severely injured and died shortly thereafter without revealing his identity. All the Gwent police knew about the John Doe was that he was Asian, or of Asian descent, and possibly Vietnamese. There was no record of him entering the country. His fingerprints provided no further information on his identity. The trail went cold.
But the police had an idea: A new, cutting edge isotope analysis technique offered the possibility that the key to the man’s identity might hang by a hair. An officer snipped a 145-millimeter-long hair from close to the man’s scalp and took it to the Stable Isotope Forensics Laboratory (now part of the James Hutton Institute) in Dundee, Scotland.
The length of the hair represented nearly 15 months worth of growth — and thus contained information on the last 15 months of the man’s life. By analyzing isotopes of hydrogen, carbon, nitrogen and oxygen in the hair, the scientists at the Stable Isotope Forensics Laboratory hoped to trace the mystery man’s most recent, and ultimately fatal, travels — including roughly when he arrived in the U.K.
Courtesy of a new isotope mapping effort, police were indeed able to track John Doe’s last 15 months. But the potential usefulness of these isotopes is even more wide-ranging. The isotope maps could help researchers solve crimes, track human geographic origins, detect counterfeit foods and drinks, identify the source of illicit drugs and follow the migration of wildlife.
About five years ago, Jason West, a scientist now at Texas A&M University, coined the word “isoscape” to describe a new kind of map: a spatial distribution of stable isotope ratios (from elements such as carbon, hydrogen, oxygen, nitrogen, sulfur and strontium) in different parts of the world, based on their known behavior in the environment. Different isotopes tell different stories. Carbon can help identify diet, for example, whereas hydrogen and oxygen, the ubiquitous components of water, can help identify provenance. The combination of different isotopic values into one map creates a far more powerful tool than a map based on any single element.
To create these maps, scientists need to collect data at many different scales. It’s a time-consuming and challenging effort, given that ratios can change from one region to the next with variations in rock, soil, hydrology and vegetation. Scientists are using a host of methods, including direct measurements of plant, soil and water types and larger-scale measurements with remote sensing instruments and laser spectroscopy to collect data on atmospheric, water and terrestrial landscapes.
To determine provenance, or anything else for that matter, using isoscapes, scientists first measure the isotopic ratios in anything from bones and hair to plants and gems, and then compare those values (which may change over time, as bones, plants and teeth grow) with an isoscape. The technique, says biochemist Helen Kemp of the James Hutton Institute, is based on a simple proposition: You are what you eat — or, in the case of the John Doe mystery, what you drink. About 30 percent of the hydrogen in a human hair comes directly from water and water-based beverages.
Drinking water generally comes from surface water sources, such as lakes and reservoirs, or from groundwater aquifers. The isotopic values of those waters are determined, in part, by the isotopic value of rain. Water, of course, is made up of hydrogen and oxygen, each of which has several isotopes. The most abundant hydrogen isotopes on Earth are protium (hydrogen-1) and deuterium (hydrogen-2); the most abundant oxygen isotopes are oxygen-16 and oxygen-18. The global “average” relationship between oxygen and hydrogen isotopes, given as the ratio of oxygen-18 to deuterium, is known as the Global Meteoric Water Line. But this relationship is only an average. In truth, these values vary greatly from place to place in waters around the world, due to local variations in temperature, altitude, climate and distance from seawater (from which the rainwater originally evaporated). That variability makes it possible to determine an isotopic signature for the water from a given place.
The first study to quantify a correlation between the hydrogen isotopic composition of modern human hairs (and nails) and local waters was published in 2007 by Wolfram Meier-Augenstein, who would later lead the scientific team that analyzed John Doe’s hair. Meier-Augenstein proposed that it was possible to determine where a person had been recently based on the hydrogen isotope record in their hair.
Strontium isotopes are another tracer of water origins — in particular, groundwater. Strontium atoms readily substitute for calcium atoms in calcium carbonate (limestone), sulfates, feldspars and other rock-forming minerals. Thus, strontium isotopes are indicators of water-rock interactions: Differences in the ratio of strontium-87 to strontium-86 reflect the influence of local aquifers on the groundwater, and thus can be used to map out regional variability in groundwater composition.
Geochemist Julian Hoogewerff of the University of East Anglia in England and his team have been working to create the first low-resolution strontium isotope geochemical map for Europe. The team has analyzed strontium from groundwater from hundreds of different European locations, finding strontium-87 to strontium-86 ratios that indicated host rocks ranging from young, mantlederived basalts to very old silicic continental crust. Combining these data with a GIS-based geological map of Europe, Hoogewerff and his team constructed a vast strontium isoscape of groundwater origins, as Hoogewerff reported at the European Geophysical Union’s annual meeting in Vienna, Austria, last April. Because the strontium in the groundwater is bio-available for uptake by plants and enters the food chain, these strontium isotopic values become reflected in, for example, honey and wheat as well as water.
The strontium map that Hoogewerff and his colleagues developed will allow the EU to trace different foods and drinks — something the EU has become particularly concerned about recently.
Concerns about the provenance of certain beverages have existed for centuries — for example, whether a sparkling wine labeled “champagne” is actually from the Champagne region in France. But with the rise of genetically modified foods and the dramatic increase in global trade, the EU has become similarly concerned about the wider food supply. Determining “food authenticity” can mean anything from uncovering the substitution of cheaper ingredients to identifying incorrect or fraudulent origins. To that end, the EU began the TRACE project (www.trace.eu.org), a five-year effort that uses analytical and geochemical tools, including stable isotope forensics, to discern the origin and journey of food products from “farm to fork.”
Much of the goal of the TRACE project is to fund expensive but necessary large-scale, commodityspecific isotope databases for food products, including water, cereals, honey, olive oil and meats. In recent years, making sure food quality is what it is purported to be — with extra virgin olive oil, for example — has also become an issue in the U.S. With these issues in mind, scientists are now engaged in constructing large-scale isotopic ratio maps of Europe, the United States and other regions, using the limited data available so far, as well as GIS and modeling.
In another fascinating case, the James Hutton Institute team has proposed a clever isotopic solution to a very serious problem that is close to the heart of Scots: using hydrogen and oxygen isotopes to detect counterfeit Scotch whisky (that is, so-called Scotch made anywhere other than Scotland). The idea, Kemp says, is that ideally, the key ingredient in Scotch whisky — Scotland’s water — would have a distinct isotopic pattern. And thus any Scotch made outside of Scotland would look quite different.
The scientists have been testing dozens of real Scotch whiskies and several fakes, but do not have enough data yet to turn this isotopic counterfeit test into a forensic test of sufficiently high specificity and selectivity. The researchers will need a much more robust database of both real and counterfeit Scotch whiskies, Kemp says.
The whisky project highlights one of the main drawbacks to forensic testing using isoscapes — the lack of sufficient data to construct robust isoscapes, which can be expensive, but are necessary. Like fingerprinting, Kemp says, it requires already existing background databases to work effectively. Fingerprints alone cannot identify a John Doe. A database matching those fingerprints to a specific person is needed before the method can work. Similarly, the isoscape method will improve dramatically with more baseline data, she says. However, for some projects still in a preliminary phase, like identifying counterfeit Scotch, a lack of sufficient data just makes it too difficult to say anything definitive about provenance.
Forensic isoscaping may still be in its early stages as a field, let alone a crime-solving tool. But at least one case has been solved with the help of forensic isoscaping: John Doe’s murder in South Wales. Once Meier-Augenstein and the team at the James Hutton Institute received the strand of John Doe’s hair, the scientists cut it into five-millimeter-long segments, each representing about two weeks of the man’s life. Focusing on the hydrogen isotopes, they analyzed the segments and compared the resulting data with known hydrogen isotopic values for waters across Europe. Ultimately, they were able to piece together a map — an isoscape — of the man’s last months, based on what he drank and ate.
His movements, they discovered, included several months spent in Eastern Europe, perhaps Ukraine, followed by a transitional period of traveling before a longer stint in Central Europe — likely Germany. Then, about three months before he died, the man arrived at the west coast of the U.K. (which includes South Wales). These finds were corroborated by additional evidence turned up by the German police, who discovered a match to the mystery man’s fingerprints: He had indeed spent some time recently in Germany.
The various lines of information ultimately led to his identification: John Doe, the police discovered, was a Vietnamese victim of human trafficking. He had been smuggled into the U.K. by a “Vietnamese Organised Crime Gang” that used the same stops across Europe identified by the hydrogen analysis as staging points on their human trafficking route. When he couldn’t repay his debt, the gang assaulted him and then dropped him at the hospital in Gwent. The police ultimately arrested, prosecuted and convicted two Vietnamese men of manslaughter.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.