
by Nate Burgess Tuesday, September 1, 2015

Stratospheric clouds facilitate ozone depletion. The ice in these clouds provides a surface on which ozone-destroying chemical reactions occur. Credit: Photograph by Lamont Poole, NASA Langley Research Center.
Each year in late September to early October, atmospheric scientists watch with anticipation as ozone concentrations over Antarctica drop, opening a window in Earth’s defenses against harmful ultraviolet radiation. This ozone “hole” grew steadily in the 1990s and set a record for its size in 2006: At its peak, the hole covered an average area of 27 million square kilometers, approximately the size of North America. But scientists think that the overall ozone layer is on the slow road to recovery, thanks to the Montreal Protocol — one of the most successful international environmental agreements in history.
The Montreal Protocol’s story begins in the early 1970s, when scientists started to worry about the effects of various industrial chemicals and air pollutants on ozone — a rare, toxic gas located primarily in the stratosphere. Although harmful at the ground level, ozone in the stratosphere is beneficial to life on Earth because it absorbs one band of ultraviolet radiation (UV-B) from the sun that can cause cancer and other health problems.
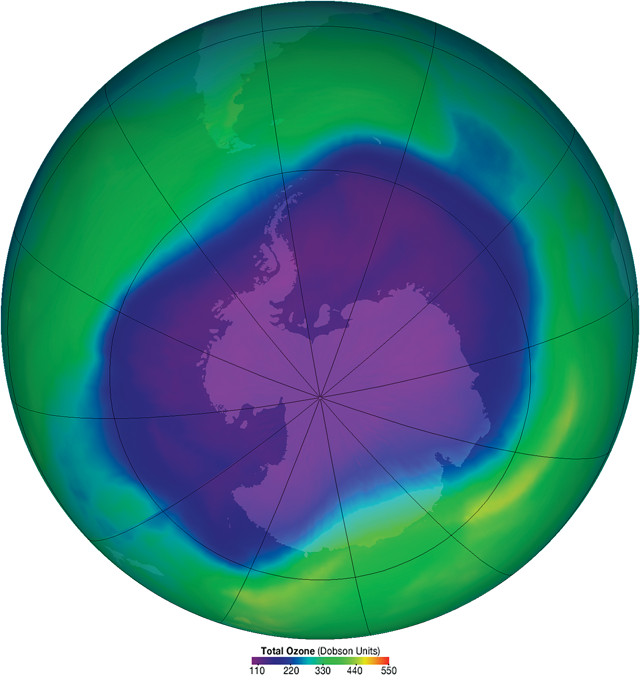
In September 2006, the hole in the ozone layer reached its largest recorded size, covering an area approximately the size of North America. Credit: NASA.
Initially, researchers suspected a link between ozone depletion and the release of nitrogen oxides from fertilizers and supersonic aircraft. Later scientists uncovered the real threat: a group of chemicals called halocarbons, especially chlorofluorocarbons, or CFCs. CFCs gained worldwide use throughout the 20th century in products like refrigerators and air conditioners, solvents and aerosol propellants.
In 1974, environmental scientist James Lovelock demonstrated that CFCs were widely distributed in the atmosphere. That same year chemists Sherwood Rowland and Mario Malino presented a hypothesis detailing the processes by which CFCs destroy ozone. They received the Nobel Prize in Chemistry for this work in 1995.
Rowland and Malino realized that CFCs pose a potential for great environmental damage because of the stability of these substances. Not easily destroyed chemically, these resilient chemicals linger in the environment long enough to diffuse into the upper atmosphere. Once CFCs reach this height, UV radiation breaks up the CFC compounds, releasing chlorine atoms. Chlorine atoms then act as catalysts, knocking off one of ozone’s oxygen atoms and converting it from O³ to O². Chlorine does this without being dragged into any stable compounds, thus remaining in the atmosphere. Therefore, scientists estimate that one molecule of chlorine can convert 100,000 ozone molecules into two-atom oxygen molecules before being removed from the stratosphere. Bromine, present in other halocarbons, has a similar, though lesser, effect.
Rowland and Malino’s hypothesis, although alarming in its global implications, was initially disputed by CFC-producing industries. Accordingly, before the United Nations considered regulating CFC emissions, it asked the U.N. Environment Programme (UNEP) to further examine ozone destruction. In March 1977, UNEP brought together experts from 32 countries to create the World Plan of Action on the Ozone Layer, which included coordinated international research and monitoring efforts.
But international efforts to regulate CFC production gained little traction until the discovery of a growing hole in the ozone layer over Antarctica in 1985. That year, a paper published in Nature summarized field data from the British Antarctic Survey that indicated that ozone levels above Antarctica during the South Pole’s spring, in September and October, dropped dramatically from the 1970s to 1985. NASA detected similar fluctuations in satellite data as far back as 1976, but discarded the phenomena as instrumental error.

Scientists in Antarctica launch a balloon carrying an ozonesonde, which measures the vertical profile of the ozone layer. Credit: NOAA Image Library.
Since the 1980s, scientists have learned that the thinning of the ozone layer is greatest over Antarctica and fluctuates seasonally because of the continent’s unique influences on the atmosphere.
During the frigid, months-long night of the South Pole’s winter, a vortex of wind surrounds the South Pole, isolating the atmosphere there from the warmer air at lower latitudes. This isolated air mass is colder than the mid-latitudes or the Arctic, so ice clouds form in the stratosphere. Chlorine- and bromine-containing chemical compounds accumulate on the ice particles. When the Southern Hemisphere’s spring arrives in September, these chlorine- and bromine-laden clouds dissipate, unleashing ozone-destroying molecules and creating a sudden decline in ozone concentrations for several months.
After the hole was discovered, world leaders and scientists sprang into action and moved forward with an agreement to regulate ozone-depleting substances. Immediate action was possible because of a resolution adopted by the Vienna Convention for the Protection of the Ozone Layer in March 1985 — just two months prior to the publication of the Nature paper. The Vienna Convention brought together 28 countries that agreed to share information on CFCs and work together to monitor and regulate the substance, if science created a clear mandate to do so. The Vienna convention also gave UNEP the power to start negotiations for a protocol to regulate ozone-depleting substances. This became the Montreal Protocol.
On Sept. 16, 1987, 46 countries signed the Montreal Protocol on Substances that Deplete the Ozone Layer. Originally, the Protocol required a 50 percent reduction from 1986 levels in the consumption and production of five major ozone-depleting substances by 1999. Since then, additional amendments have added many other chemicals to the list. As of 2008, the Montreal Protocol controlled 96 chemicals. These chemicals will be reduced and phased out in developed countries on chemical-specific timelines during the next 20 years. Developing countries have an additional grace period, with the last chemicals phased out by 2040.
These regulations have worked: UNEP estimated that 96 percent of all ozone-depleting substances regulated by the protocol were no longer used globally by 2006. Much of the remaining consumption occurs in developing countries, where implementation of the protocol has been slower.
The World Meteorological Organization Global Ozone Research and Monitoring Project’s 2006 assessment of the state of the ozone was also positive: Total abundance of anthropogenic ozonedepleting substances in the atmosphere decreased by 8 to 9 percent relative to their estimated peak in 1992 to 1994. Scientists attribute this decline to the removal of the chemicals with shorter lifespans from the atmosphere, such as methyl chloroform and methyl bromide. Because of this removal, global ozone levels are now stabilized and no longer declining.
It will take longer for ozone levels to fully rebound, however. Best estimates by the World Meteorological Organization indicate that global ozone levels will not return to pre-1980 levels for another 40 years or so, after the longer-lived ozone-depleting substances finally leave atmospheric circulation and ozone creation again outpaces ozone destruction. The ozone hole will persist even longer: Recovery to pre-1980 ozone levels in Antarctica is not expected until sometime between 2060 and 2075.
Things would be a lot different without the Montreal Protocol. By the year 2050, UNEP estimates, the ozone layer would be 10 times thinner than with current regulation. This level would result in twice as much UV-B radiation reaching Earth in the highly populated mid-northern latitudes, causing more than 20 million more cases of cancer and 130 million more cases of eye cataracts. In short, while the ozone layer is still a matter of worldwide concern, the Montreal Protocol’s success has greatly reduced the consequences of humangenerated ozone destruction.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.