
by Carolyn Gramling Thursday, January 5, 2012
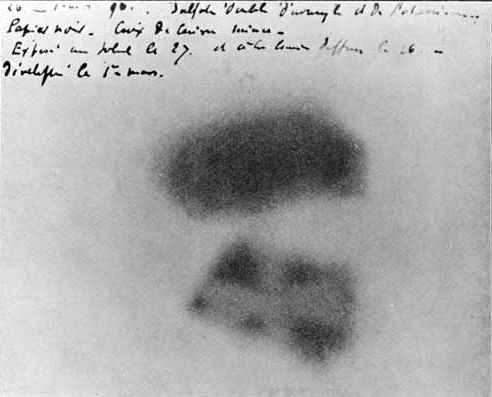
A photographic plate made by Henri Becquerel shows the effects of exposure to radioactivity. A metal Maltese cross, placed between the plate and radioactive uranium salt, left a clearly visible shadow on the plate. Henri Becquerel
February 26, 1896, was an overcast day in Paris — and that presented a problem for French physicist Antoine Henri Becquerel. Becquerel was hoping to demonstrate a link between minerals that glow when exposed to strong light and a new type of electromagnetic radiation called X-rays. The weather thwarted this experiment — but that failure inadvertently produced an entirely new discovery: natural radioactivity.
Becquerel was interested in the phenomenon of fluorescence, in which some materials glow when exposed to sunlight. Physicist Wilhelm Röntgen had recently discovered X-rays; Becquerel thought the two phenomena might be connected, and had designed an experiment of his own. He planned to expose a fluorescing material to the sun, and then place it and a metal object over an unexposed photographic plate. If the developed plate showed the image of the object, he concluded, that would suggest that fluorescing materials are actually emitting X-rays.
But the next day was cloudy as well, and Becquerel was forced to postpone his experiment. He wrapped his fluorescing crystals — a uranium compound called potassium uranyl sulfate — in a black cloth, along with the photographic plate and a copper Maltese cross, and waited for a sunnier day.
Several days later, when Becquerel finally removed the plate from the drawer, he discovered to his surprise that a distinct image of the cross appeared on the plate — although it had never been exposed to sunlight. The only conclusion was that the crystals themselves were emitting radiation. Excited by this prospect, Becquerel decided to repeat the conditions of his unintentional experiment: He again placed a crystal of uranium salt on a photographic plate; he also experimented with putting a crystal on a photographic plate with a sheet of aluminum between, and with a sheet of glass.
After being placed in the dark for several hours, all three plates were blackened by radiation (the crystal in direct contact with the plate showed the strongest blackening). “I am now convinced that uranium salts produce invisible radiation, even when they have been kept in the dark,” he wrote in his diary of his experiments.
This discovery of spontaneous “radioactivity” (a term coined by Becquerel’s doctoral student, Marie Curie) eventually earned Becquerel a Nobel Prize for Physics in 1903, which he shared with Marie Curie and her husband Pierre Curie.
Becquerel came from a family of scientists: His grandfather, Antoine César Becquerel, had discovered piezoelectricity (the electrical charge that accumulates in crystals and other materials as a result of applied mechanical strain). His father, Alexandre-Edmond Becquerel, had invented the phosphoroscope, a device that measures how long a phosphorescent material continues to glow after removing the source of light.
Becquerel spent a lot of time in his father’s laboratory, and he was initially interested almost exclusively in optics. When he became a research physicist, he embarked on his own study of the radiation of light: He explored how magnetic fields polarized light, how infrared light produced phosphorescence in some materials and how crystals absorb light. Upon his father’s death in 1891, Becquerel succeeded to his father’s two chairs, one a Chair of Physics at the Conservatoire National des Arts et Métiers and the other a Chair of Physics at the Muséum National d’Histoire Naturelle, both in Paris.
His research took a new turn when he attended a lecture on X-rays at the Académie des Sciences in Paris. In January 1896, the French mathematician Jules-Henri Poincaré had received a letter from Röntgen, which contained several surprising photographs that showed the outline of bones within a hand. In the letter, Röntgen explained that the images had been taken with a new discovery, the X-ray. Poincaré was astonished, and reproduced the images himself. Poincaré presented his own images at the Académie two weeks later, to enthusiastic response.
Becquerel was in the audience that day, and wondered whether there was any connection between the ghostly X-ray images and the phenomena of fluorescence and phosphorescence that he and his father had studied. Becquerel had already studied the phosphorescence of uranium salts in particular and was familiar with photography, so he decided to undertake his own experiments on the subject of X-rays.
On Feb. 24, 1896, Becquerel presented his initial results to the Académie des Sciences: His phosphorescing uranium salts, after exposure to sunlight, had left faint images on several photographic plates. But the smudgy images were far less intriguing than the sharp X-ray images shown a few weeks earlier, and Becquerel resolved to try again. He prepared new arrays of crystals and photographic plates, and decided that he needed very strong sunlight to produce the best images.
But nature didn’t cooperate; Becquerel didn’t get his sunny day. Still eager to show something to the Académie, he took the plates and the crystals out of his drawer. He expected to see more of the same faint images, but was startled to find instead crisp silhouettes of his metal objects, including the Maltese cross. Stimulation of the crystals by sunlight before or during the experiment, it seemed, was not necessary to produce the images — suggesting that the crystals themselves were emitting radiation, without external stimulation. On March 1, 1896, Becquerel presented the discovery of spontaneous radioactivity to the Académie.
The discovery of spontaneous radioactivity spread rapidly and engendered a flurry of new research on the phenomenon, much of it by Marie and Pierre Curie. Becquerel also continued to study the phenomenon: In 1899, he discovered that X-rays could be deflected by a magnetic field, suggesting that the radiation contained electrically charged particles. The international unit of radioactivity, the becquerel (defined as one nucleus decay per second), was named for him. But Becquerel was still fascinated by the interaction between crystals and light, and he eventually returned to this research, studying how crystals absorb and polarize light.
Meanwhile, Marie Curie took on the study of uranium rays for her thesis research. While studying the uranium-bearing minerals pitchblende and chalcolite, she discovered that in addition to uranium, other elements emitted the “Becquerel rays”: thorium and a powerfully radioactive element that Curie dubbed “radium.”
The discovery of radioactivity had profound impacts on chemistry and physics at the time. The powerful radiation, including heat, spontaneously emitted by radium seemed to contradict the law of conservation of energy: What was the source of that energy? Physicists began to reconsider the structure of the atom, and ponder whether some change in the atom itself could be responsible.
In 1899, physicist Ernest Rutherford discovered that these materials actually emit different types of radiation (alpha, beta and gamma rays), defined by their penetrating power. A decade later, Rutherford proposed a model of the atom in which a small, dense nucleus of protons was surrounded by orbiting electrons — and later demonstrated that the source of the radioactivity was the spontaneous disintegration of this atom, thereby “transmuting” the element into another element. In 1919, Rutherford — now known as the father of nuclear physics — published a paper that detailed “splitting an atom”; he had succeeded in forcing protons out of the nucleus, the first step to the 1938 discovery of nuclear fission.
Becquerel died only 12 years after his discovery of radioactivity, at age 54. Although his cause of death was unspecified, he had developed serious burns on his skin, likely from the handling of radioactive materials. A few decades later, Marie Curie died of aplastic anemia, likely from exposure to radiation without proper safety measures. The damaging effects of ionizing radiation were still unknown at the time.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.