
by Timothy Oleson Friday, August 8, 2014
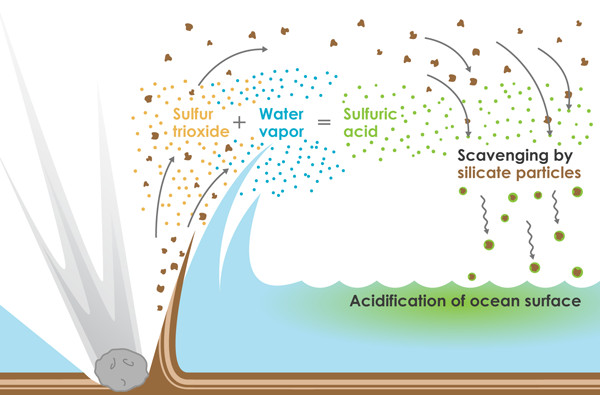
The proposed mechanism by which the oceans could have been acidified within days after the Chicxulub impact 65.5 million years ago. Credit: Kathleen Cantner, AGI.
Within days after the massive Chicxulub impact that ended the Cretaceous Period 65.5 million years ago, a deluge of acid rain may have turned Earth’s ocean surfaces into suddenly inhospitable homes for a multitude of microorganisms, ultimately pushing them to extinction, according to a new study.
The acid rain, the research suggests, could have resulted from the rapid conversion of trillions of kilograms of aerosolized sulfur — kicked into the skies when the impact struck sulfate-rich sediments in the Yucatán Peninsula — into sulfuric acid. The surge of sulfuric acid would have then been flushed from the atmosphere into the ocean by debris falling back to Earth, overwhelming the ocean’s natural neutralizing ability, driving acidity up and concentrations of dissolved carbonate down by a factor of roughly 100 at the ocean surface. It would have been a toxic turn of events for microorganisms like foraminifera and algae, along with larger animals like ammonites, which harvest calcium and carbonate from seawater to build their skeletons.
The impact-acid rain explanation for the end-Cretaceous pattern of extinction among marine plankton — which saw surface-dwelling species die off more than deepwater species, and calcifying organisms fare poorly compared to those that use other materials to build shells — has been proffered before. But scientists have been unable to understand how it actually might have happened. That’s partly because many studies have assumed that the sulfur blasted from the surface would have been mainly sulfur dioxide, and that much of it would have remained in the atmosphere for weeks or months after the impact, even after converting to sulfuric acid. Had this scenario occurred, acid rain would have fallen into the oceans gradually and would have been easily neutralized.
However, in the recent study published in Nature Geoscience, researchers fingered a different sulfur-bearing culprit. Far more so than sulfur dioxide vapor, “sulfur trioxide-rich impact vapor was released during the Cretaceous-Paleogene impact, [likely resulting] in the sudden onset of globally extensive acid rainfall and severe ocean acidification,” says lead author Sohsuke Ohno of the Chiba Institute of Technology in Japan.
Ohno and his colleagues recreated the impact experimentally by firing tiny metal projectiles at speeds of up to 25 kilometers per second at targets made of anhydrite, a calcium sulfate mineral found in the sediments of the Yucatán Peninsula. They then measured the composition of the vapor released by the target, using a mass spectrometer to distinguish the different sulfur oxides. Each time they ran the experiment, sulfur trioxide concentrations were roughly 100 times higher than sulfur dioxide concentrations, suggesting that high levels of sulfur trioxide, which reacts quickly with water vapor to form acid, would have permeated the skies in the actual event.
The team then modeled what the immediate aftermath would have looked like in both the atmosphere and oceans, factoring in massive quantities of pulverized silicate rock that were also thrown far into the sky. As this rocky dust quickly settled back toward the surface, the much smaller droplets of sulfuric acid, which otherwise would have remained aloft longer, would have stuck to the silicate particle surfaces and hitched a ride back to the surface.
A big issue in discussions of ocean acidification occurring after the impact has been “that not only do you have to take all of this sulfur out of the atmosphere and throw it back to Earth … but you have to do it very quickly,” says Ellen Thomas, a paleoceanographer at Yale University who was not involved in the work. Scavenging of sulfuric acid by silicates hadn’t been thoroughly considered in earlier models, Thomas says, and “it makes the [sulfuric acid] fall out of the atmosphere much more rapidly than previously thought.”
The result at the ocean surface would have been a rapid decrease of about two pH units (equivalent to a 100-fold increase in acidity), Ohno says, which “would have been severe for carbonate-secreting plankton species.” The acidity would have gradually been alleviated by mixing between surface and deeper waters, but it would take at least a few years to return to pre-impact conditions, he says.
Thomas notes that there could have also been contributors to acid rain other than sulfur, such as nitrogen oxides, and that it’s still not clear what exactly happened during and after the Chicxulub impact. But, she says, the extinction pattern that likely would have resulted from the scenario presented in the new study agrees well with what is observed in the paleoceanographic record. The work “could lead to a lot of other interesting new research.”
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.