
by Josh Knackert Thursday, December 14, 2017

NASA astronaut Peggy Whitson holds the American record for the longest cumulative time spent in space at 665 days. Bone loss is a serious concern for space travelers — in less than half a year, they can lose up to a third of what a nonspace-traveler would expect to lose in a lifetime — so NASA monitors the bone density of astronauts. Credit: NASA.
Astronauts lead dangerous lives. Besides the inherent risks of blastoff, re-entry and space flight, astronauts experience myriad physical changes from spending time in microgravity. They’re frequently monitored for kidney stones, for example, and for muscle and bone loss that result from a prolonged lack of gravity. How to combat the bone deterioration seen during extended time in space is one of the key questions NASA hopes to resolve before sending people on longer missions, such as to Mars.
But bone deterioration isn’t just a problem for astronauts. “Osteoporosis threatens half of Americans over age 50,” says Ariel Anbar, a geochemist at Arizona State University. A number of other diseases, including genetic, digestive and blood disorders, along with certain drugs and chemotherapy and radiation treatments, also cause osteoporosis. Currently, evaluation of bone loss relies heavily on bone density scans that have disadvantages. For example, scans have to be repeated over time to monitor changes, which exposes patients to increased doses of radiation from X-rays, and it typically takes months of bone loss before a change in density can even be distinguished in scans. For NASA, this prolonged timeline, and the physical space and resources needed to equip the International Space Station or spaceships with bone density scanners, make the technique especially impractical.

Among the efforts astronauts make to stay healthy in space are exercises intended to maintain bone health. Credit: both: NASA.
What if, instead, urine or blood samples could tell doctors a patient’s bones were deteriorating well before a bone scan could? In an interdisciplinary collaboration, Anbar and NASA nutritionist Scott Smith are working to make this a reality. The key lies in measuring calcium: not simply how much is present in these bodily fluids, but also the ratios of different calcium isotopes. Together, they have measured concentrations of calcium isotopes — specifically stable isotopes, which occur naturally and do not decay into other elements — in the urine of participants in a long-term bed-rest study run by NASA to mimic conditions seen in microgravity and study the effects on human anatomy. The bone deterioration that occurs in space and during bed rest is similar to the deterioration that results from bone diseases like osteoporosis. Thus, the team’s research could have significant implications for the diagnosis and treatment of a variety of serious diseases in addition to its relevance for space travel.
Stable isotope techniques like these were developed by geoscientists, who have relied on them for decades, but they’ve only recently been applied in studies of human health. And besides showing promise for determining bone loss, stable isotopes may be useful as biomarkers for a variety of other health problems.

Astronauts' health is constantly monitored. Here, Japan Aerospace Exploration Agency astronaut Akihiko Hoshide gets a blood draw at the International Space Station. Credit: NASA.
Isotopes are versions of the same element with different numbers of neutrons, meaning each isotope has a different atomic weight. Calcium atoms, for example, can have between 14 and 37 neutrons, meaning there are 24 possible isotopes; however, only six are considered stable isotopes. These stable isotopes occur in nature in various ratios depending on the setting and on fractionation processes that naturally separate isotopes from one another. Thus, isotopic ratios can serve as geochemical signatures that researchers can use to learn about where and when a material originated or how it’s changing.
“The beauty of isotopes is that they can compress complexity,” says Joseph Skulan, a geochemist and biologist at Elemental Biomarkers, LLC, in Lodi, Wis. “Even a few isotope values can provide a lot of information about a system,” he says. Geologists and geochemists first recognized that isotopic signatures were present and predictable in a number of inorganic systems in the 1940s. And for more than 70 years since, the geosciences have relied on stable isotopes to evaluate everything from past and present climates to the diets of ancestral and modern humans.
For the first 50 years, stable isotope geochemistry revolved around only a handful of low-mass, or light, stable elements: hydrogen, carbon, nitrogen, oxygen and sulfur. Researchers evaluated how these elements cycled through the atmosphere, oceans and soils over geologic time using isotopic signatures locked in rocks of different ages.
Scientists are testing whether they can determine calcium levels and potential bone loss via a urine or blood sample. Credit: Gwyn Gordon.
Biologists began evaluating stable isotopes in live plant samples in the 1960s, with early studies focusing on fractionation patterns in carbon isotopes resulting from photosynthesis. While various other potential applications for stable isotope science in biology quickly became apparent, there was an early roadblock: Biological and ecological studies require evaluation of a significantly larger number of samples than most geology studies.
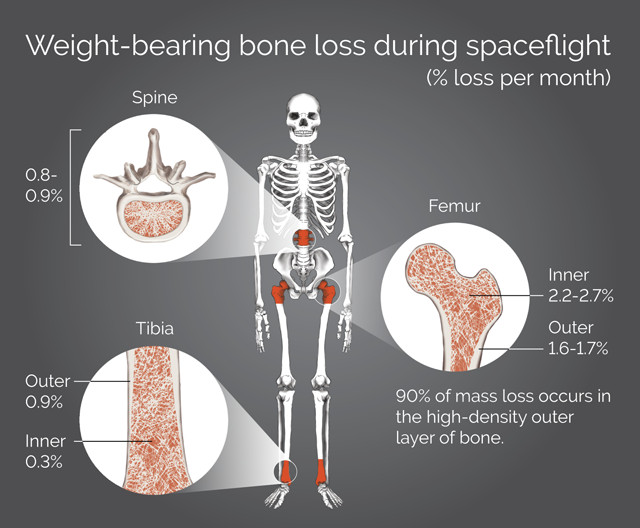
Astronauts lose bone density from the lack of weight on the skeleton, known as skeletal unloading. Credit: K. Cantner, AGI.
“Stable isotope science in biology took so long to develop because the sample prep was slow and tedious,” says Craig Stricker, a research biologist with the U.S. Geological Survey in Denver, Colo., who analyzes stable isotopes in wildlife and ecology studies. But “increased automation of sample processing in the ‘90s revolutionized stable isotope applications for biology,” he says. Additionally, the emergence of a new method for evaluating atomic weights allowed researchers to analyze isotopes from heavier elements — including calcium, iron, nickel, copper and zinc — opening new avenues for research. A 2014 review of stable isotope geoscience in the journal Chemical Geology describes the past several decades as a “sustained burst of innovations and discovery as dramatic as any period since [the field’s] foundation in the 1940s and ‘50s.” This newfound analytical potential greatly diversified possible applications of stable isotope science, spawning new subdisciplines in geology, biology, chemistry and forensics.
In the mid-‘90s, Skulan, then a biology and geochemistry graduate student at the University of California, Berkeley, recognized the untapped potential of using calcium isotopes in biology. Skulan, with Berkeley colleagues Donald DePaolo and Thomas Owens, published one of the first studies evaluating stable calcium isotopes in a biological system, looking at their fractionation in the skeletons of various marine organisms.
As the techniques evolved in biology — allowing for stable isotope studies of bone, then soft tissue, then bodily fluids — it became apparent that isotope fractionation in the human body was a field ripe for study. “A lot of what happens in the human body is inorganic chemistry,” Skulan says. “There is enormous potential there.” Wildlife and medical researchers suggest that if changes in a human’s or animal’s body can be monitored via stable isotope methods, then isotopic ratios may likely be useful as biomarkers of nutrition, toxicity and disease.
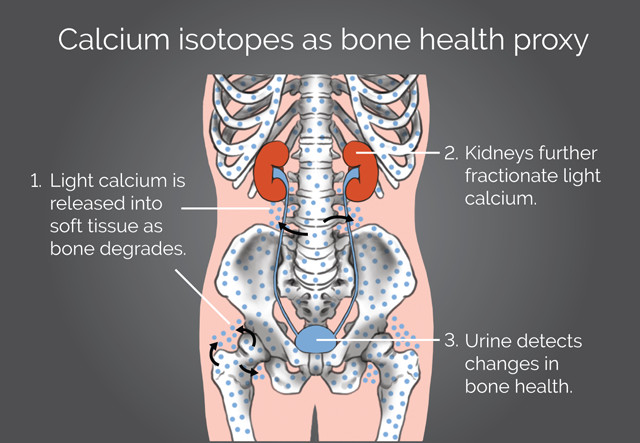
Researchers hope to use calcium isotopes in urine samples to track bone loss, even before traditional bone density scans can detect it. Credit: K. Cantner, AGI.
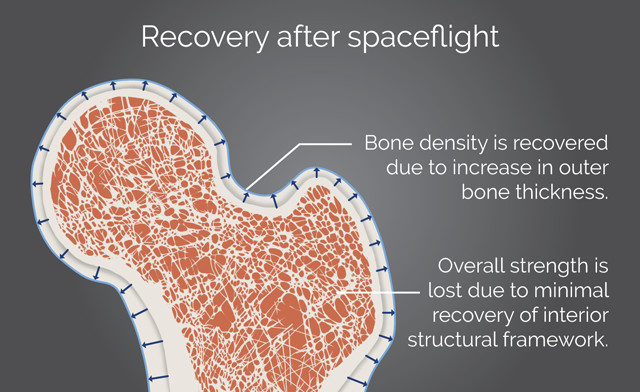
Even though bone thickness can be recovered after spaceflight or bed rest, lost bone strength may be irrecoverable due to internal restructuring of bone tissue. Credit: K. Cantner, AGI.
By the mid-2000s, Skulan and Anbar began collaborating to study calcium isotopes. In their first study, published in Clinical Chemistry in 2007, they measured calcium isotopes in the urine of patients participating in the NASA bed-rest study supervised by Smith. The team reported the first correlation between bone mineral balance and calcium isotopes in the human body. NASA then funded a number of follow-up studies. “The NASA funding has been essential,” Skulan says, emphasizing that it facilitated these early studies, which established the reputability for using stable calcium isotopes to study changes in bone. These studies then garnered attention from a number of clinicians and biomarker researchers.
Currently, isolation and measurement of calcium isotopes from biological fluids like urine or blood include several complicated and time-consuming steps, involving sample preparation and the use of column chromatography and mass spectrometry. The initial sample preparation alone takes more than 12 hours and must be performed in a clean environment, because even trace amounts of calcium contamination from the surrounding air or surfaces could throw off the measured isotopic ratios.
Still, these current methods, developed by Skulan, Anbar and their colleagues, represent a significant improvement over the last decade, the researchers say, increasing the number of samples that can be processed without sacrificing accuracy. And there is potential for automating certain steps, further shortening the process. Such improvements are important if calcium isotopic measurements are to become practical for use in clinical settings, Anbar says.
In the 2007 study, Skulan, Anbar, Smith and other collaborators reported that lighter calcium isotopes are more concentrated in the urine of patients experiencing bone deterioration. They found this by measuring the ratio of two of the three most common stable calcium isotopes found naturally, the lighter calcium-42 isotope with 22 neutrons compared to the heavier calcium-44 isotope with 24 neutrons. During bone formation, lighter calcium isotopes are preferentially incorporated into the bone, but as bone deteriorates, this stockpile of light calcium isotopes leaches into the surrounding soft tissue, blood and eventually into urine, shifting calcium isotopic ratios relative to healthy patients not experiencing bone deterioration.
In a follow-up study in Proceedings of the National Academy of Sciences in 2012, the team found that bone deterioration could be detected via calcium isotopes in urine after only one week of bed rest, dramatically abbreviating the timeline for detection. “Bone density scans tell you the past; we want to know how the bones are changing right now,” Anbar says. “Calcium isotopes offer a current snapshot.” After the promising results observed in urine, the researchers wondered whether blood offered similar detection potential, and if changes in calcium isotopes progressed similarly in astronauts experiencing bone loss from prolonged exposure to microgravity.
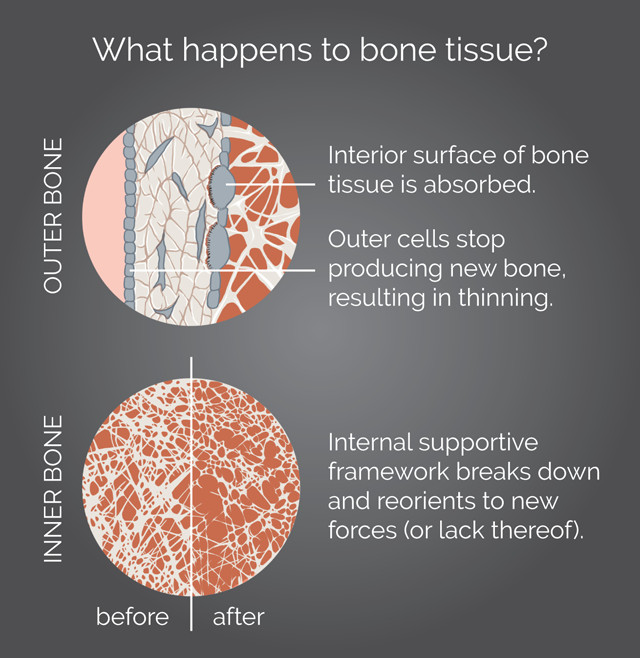
How bone tissue breaks down over time, a process that happens more rapidly in astronauts and bed-rest patients. Credit: K. Cantner, AGI.
The first question was answered in a study published in the journal Bone in 2015 in which the team found that calcium isotope changes seen in blood mimicked those observed in urine. This is especially relevant for clinical applications in which blood draws are already a common occurrence. The researchers expect to soon publish the results of another round of experiments designed to answer their second question, Anbar says. “Urine from astronauts is an ideal test case — they are constantly monitored, otherwise healthy, and will experience bone loss due to their time in microgravity,” Anbar says. The process by which astronauts lose bone density from the lack of weight on the skeleton, also known as skeletal unloading, may differ from what happens in patients in the bed-rest studies, he says. This could offer unique insights into how bone loss can vary in different environments, providing helpful data for clinicians.
Medicine currently lacks sensitive, specific biomarkers for bone mineral balance — the balance between bone formation and breakdown. The research so far indicates that measurements of calcium isotopes could improve bone loss monitoring and sidestep some downsides associated with current techniques. Isotope ratios are likely more sensitive than bone density scans, and there’s no associated radiation. Clinicians would greatly benefit from earlier and more detailed detection of bone loss, since doctors could begin interventions sooner and potentially even customize treatment for patients based on their individual rates of bone loss, says Rafael Fonseca, a physician-researcher at the Mayo Clinic in Phoenix, Ariz. His lab studies the disease multiple myeloma, a cancer that causes bone loss.

On spaceflights to Mars or elsewhere in the solar system, astronauts will be confined to microgravity and cramped positions for far longer periods than previously experienced. As such, NASA is conducting bed-rest studies to determine how bone loss and other health issues may affect people, including astronauts, during extended periods of inactivity. Credit: all: NASA.
As this body of research has progressed and established the potential of calcium stable isotopes, clinicians studying a number of different diseases have taken interest. “Proof of concept is there unequivocally,” Fonseca says. “The field sorely needs a biomarker for bone loss to inform disease progression and treatment response,” he adds. “Currently, pain is often the first sign of bone issues in a myeloma patient, and scans primarily look for localized, destructive lesions. Stable isotope measurement offers the promise of a global reading.”
In 2014, Skulan, Anbar, Fonseca and other collaborators published a small, preliminary study in the journal Leukemia in which they studied blood from multiple myeloma patients. As expected, blood from patients with an active form of the cancer was enriched in lighter isotopes compared to patients whose cancer was currently inactive, suggesting the bone loss associated with active myeloma had shifted the calcium isotope fractionation. The results offered hope that multiple myeloma, like osteoporosis, could be monitored using calcium isotopes. And there are likely many other diseases for which calcium isotopes may prove useful as well.
However, a number of physical and logistical hurdles still need to be overcome before clinical applications of calcium stable isotope methods can be realized, Fonseca says. “Efforts to streamline the [sample preparation] process for NASA applications directly lends itself to medicine,” Fonseca says, but he notes that the process must still be simplified and accelerated to be effective for diagnostic purposes. Anbar and the researchers in his lab are hoping to overcome some of these limitations. They are attempting to measure calcium using laser spectroscopy, which, compared to current calcium stable isotope spectroscopy methods, would be cheaper, quicker, simpler, and require less lab space. All of these help move researchers closer to achieving clinical application, Fonseca says.
Another barrier is that baselines for biomarkers need to be established, Fonseca says: To be effective, clinicians first need to know how biomarkers behave in a healthy individual. Before blood sugar could be used as a biomarker for diabetes, for example, researchers had to establish what the healthy range of blood-sugar levels was among a diverse global population, as well as the extent to which levels could be changed by the disease. Stable isotopes currently lack such established baselines — a crucial next step before doctors can begin to develop relevant therapies.
“Larger studies are needed to validate [stable isotopes] as clinically useable biomarkers,” Fonseca says, noting also that different baselines should be established for different tissues and organs, as well as for the whole body, to provide doctors with the range of healthy values present in the general population. Once established, he says, these baselines could also be compiled into a centralized database, similar to how GenBank, a database of all publically available DNA sequences, compiles genetic information and metadata to inform studies across an array of scientific fields.
While calcium isotope applications in medicine may still require some key advances before they’re used for clinical purposes, the potential demonstrated so far is helping lower the barrier for the use of other stable isotope geoscience applications in medicine, Skulan says. “Initially, it was difficult to get these studies into medically relevant journals, or catch the attention of clinicians,” he notes. “Now that the calcium application has an established foundation, similar studies are more readily accepted and receive far more recognition.”

Researchers at the Anbar Lab are working on improving the process to collect calcium isotopes from urine and blood to detect bone deterioration. Credit: Gwyn Gordon.
Carbon stable isotopes are currently being investigated in a phase II clinical trial on patients in intensive care units in five different states in the hope that they may signal impending septic infections. The key difference in this application is that it is gas-based: Carbon isotopic concentrations are measured in the carbon dioxide exhaled by patients. Isotopic compositions in gases are far easier to evaluate than in liquids or solids, and the associated techniques are far more advanced. Carbon is also a light element whose isotopes have been studied far longer than calcium, meaning that their behavior and baselines are more established.

The machines used to measure calcium isotopes in blood or urine, such as those seen here, are too large to be carried into space and used by astronauts. Researchers are working to simplify such analyses to reduce the cost, time and lab space they require. Credit: Gwyn Gordon.
A number of heavy element isotopic applications are also beginning to show promise. Iron stable isotopes, for example, may inform not only iron metabolism in the body, but also liver diseases and anemia. Liver diseases and cancers — including breast, liver and colon cancers — cause enrichments of lighter copper stable isotopes in blood, although the mechanism(s) responsible for this shift are unknown. Preliminary research also hints at light zinc isotopes concentrating in breast cancer tumors, providing yet another possible stable isotope cancer biomarker. These and other examples of fractionation of metal stable isotopes in the human body hint that ratios of these isotopes may shift predictably under disease conditions, says Anna Barker, co-director of the National Biomarker Development Alliance at Arizona State University. “Metal levels are closely controlled throughout [the history of human] evolution and are essential to physiology,” Barker says. “Even small changes in metal concentrations likely signal problems in the human body.”

If we want to send humans to Mars someday, we have to figure out how to keep them safe, and that includes keeping their bones from deteriorating too much. Credit: NASA/JSC/Pat Rawlings, SAIC.
The expanding body of nontraditional stable isotope research has even played a part in spawning a new field called metallomics — the study of metals (and elements that can behave like metals) distributed throughout the body. These elements can occur as both free ions and bound in proteins called metalloproteins (a well-known metalloprotein is hemoglobin, which transports oxygen in the blood and features iron as an integral component).
Barker sees immense potential in stable isotopes for biomarker discovery. “There are so few biomarkers currently available for disease diagnosis, especially in cancer,” she says. Only a few biomarkers are widely used across all of medicine, she says. The need for more is great.
© 2008-2021. All rights reserved. Any copying, redistribution or retransmission of any of the contents of this service without the expressed written permission of the American Geosciences Institute is expressly prohibited. Click here for all copyright requests.